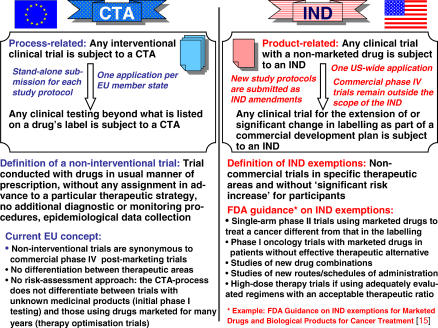
Figure 2. Comparison of the EU CTA and the US IND Application Procedures.
For noncommercial, patient-focused research, supplemental guidelines were issued in the US, whereas in the EU, exemptions from the clinical trial application (CTA) process and applicable GCP principles are restricted to post-authorisation safety studies. Observational studies using epidemiological methods are generally exempted from regulation in the EU and US.