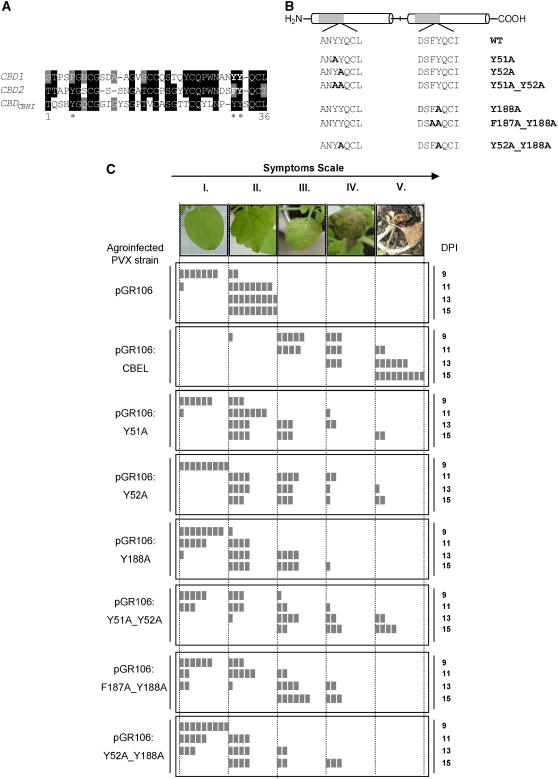
Figure 2.
Site-Directed Mutagenesis Analyses of CBEL Using the PVX Expression System.
(A) Alignment of CBD1 and CBD2 of CBEL and the cellobiohydrolase I CBD from T. reesei. The deduced amino acid sequences of CBDs from CBEL (CBD1, CBD2) and cellobiohydrolase I CBD from T. reesei (CBDCBHI) were aligned according to the ClustalW and BoxShade sequence analysis software programs. Single-letter codes are used for amino acid residues, black or gray boxes indicate identical or similar residues, and dashes indicate gaps introduced to allow optimal alignment of the sequence. Asterisks mark the positions of the aromatic amino acids of the CBD from T. reesei implicated in cellulose binding affinity.
(B) Scheme indicating the positions of mutations introduced into the CBEL sequence. Y51A, Y52A, and Y188A correspond to a single amino acid exchange of Tyr-51, Tyr-52, and Tyr-188 with Ala, respectively. Y51A_Y52A, F187A_Y188A, and Y52A-Y118A are double point-mutated versions of CBEL.
(C) Comparative analysis of necrosis-inducing activity of the mutated versions of CBEL. At top is the symptom scale used in the experiments, corresponding to the following phenotypes: no symptoms (I), typical mosaic disease symptoms (II), localized necrosis (III), confluent or spreading necrosis (IV), and death of the plant (V). Observations were conducted every 2 d from 9 to 15 d after inoculation (DPI). Control plants were inoculated with A. tumefaciens harboring the empty PVX expression vector (pGR106). Test plants were inoculated with agroinfected PVX strain containing a wild-type (pGR106:CBEL), point-mutated (pGR106:Y51A, pGR106:Y52A, pGR106:Y188A), and double mutated (pGR106:Y51A_Y52A, pGR106:F187A_Y188A, pGR106:Y52A_Y188A) DNA construct. Each box symbolizes one inoculated plant.