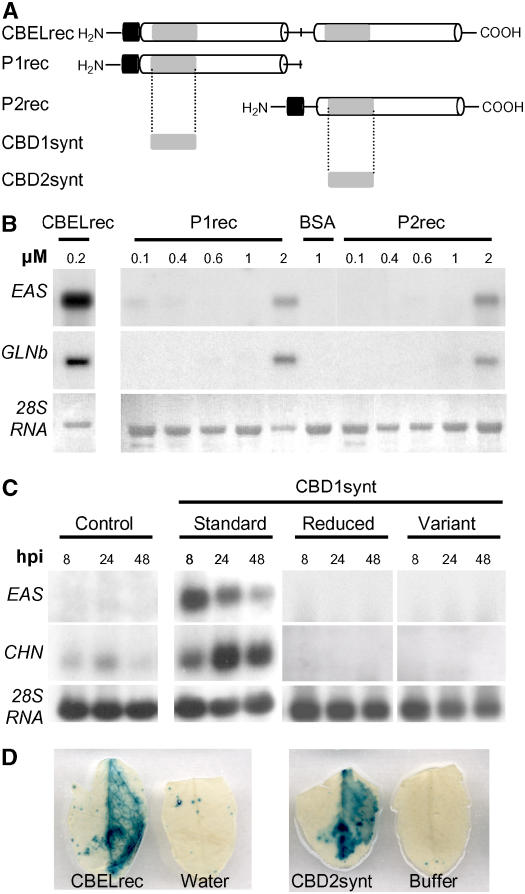
Figure 6.
Elicitor Activity of CBDs.
(A) Schemes of CBEL moieties tested for elicitor activity. CBELrec, P1rec (amino acids 1 to 144), and P2rec (amino acids 144 to 268) were produced in E. coli. CBD1synt (amino acids 5 to 41) and CBD2synt (amino acids 142 to 177) were synthesized chemically. The two direct Cys-rich repeats are represented as white boxes and separated by a line corresponding to the Thr/Pro linker containing a central Met. Gray boxes symbolize the CBD subdomains, and the black boxes indicate the FLAG peptide localized at the N-terminal part of the recombinant proteins.
(B) Dose response of defense gene expression encoding sesquiterpene cyclase (EAS) and basic glucanase (GLNb) in tobacco leaves upon treatment with CBELrec, P1rec, and P2rec. Total RNA was extracted at 24 h after inoculation with P1rec (0.1 to 2 μM), P2rec (0.1 to 2 μM), CBELrec (0.2 μM), and BSA (1 μM). For the RNA gel blot analyses, equal loading of the gels was checked on the membranes by visualization of rRNAs (28S RNA) under UV light (λ = 254 nm).
(C) Tobacco leaf infiltrations were performed with 5 μM solutions of the synthetic peptide CBD1synt (standard), peptide CBD1synt preincubated in 50 mM DTT (reduced), peptide CBD1synt in which Cys residues were replaced by Ser residues (variant), or 250 μM sodium phosphate buffer as a control. Total RNA was isolated from tobacco leaves at 8, 24, and 48 h after inoculation (hpi) after treatment and analyzed for expression of defense genes encoding sesquiterpene cyclase (EAS) and chitinase (CHN). For the RNA gel blot analyses, equal loading of the gels was checked on the membranes by visualization of rRNAs (28S RNA) under UV light (λ = 254 nm).
(D) PR1–β-glucuronidase (GUS) A. thaliana leaves were infiltrated with 0.2 μM CBELrec or a 5 μM solution of the synthetic peptide CBD2synt as indicated. GUS activity was revealed at 48 h after inoculation.