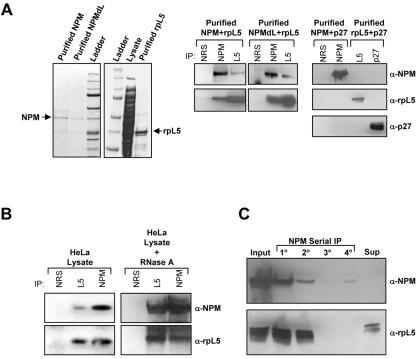
FIG. 5.
NPM interacts directly with rpL5. (A, left) Recombinant NPM, NPMdL, and rpL5 were purified from bacterial lysates using Ni-nitrilotriacetic acid affinity chromatography. Purified proteins were separated by SDS-PAGE and detected with Coomassie blue stain. (A, middle) Purified NPM or NPMdL proteins were incubated overnight with rpL5 and immunoprecipitated (IP) with NRS or antibodies recognizing NPM or rpL5. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM and rpL5 antibodies. (A, left) Purified NPM or rpL5 proteins were incubated overnight with recombinant p27 and immunoprecipitated with NRS or antibodies recognizing NPM, rpL5, or p27. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM, rpL5, and p27 antibodies. α, anti. (B) Proteins from HeLa cell lysates were immunoprecipitated with NRS, rpL5 antibody, or NPM antibodies. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM and rpL5 antibodies. Alternatively, HeLa lysates were pretreated for 1 h with RNase A prior to immunoprecipitation as described above. (C) HeLa lysates were subjected to serial immunoprecipitation with NPM antibodies (lanes 1o to 4o). Precipitated proteins and proteins in the final supernatant (unbound) were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies recognizing NPM and rpL5.