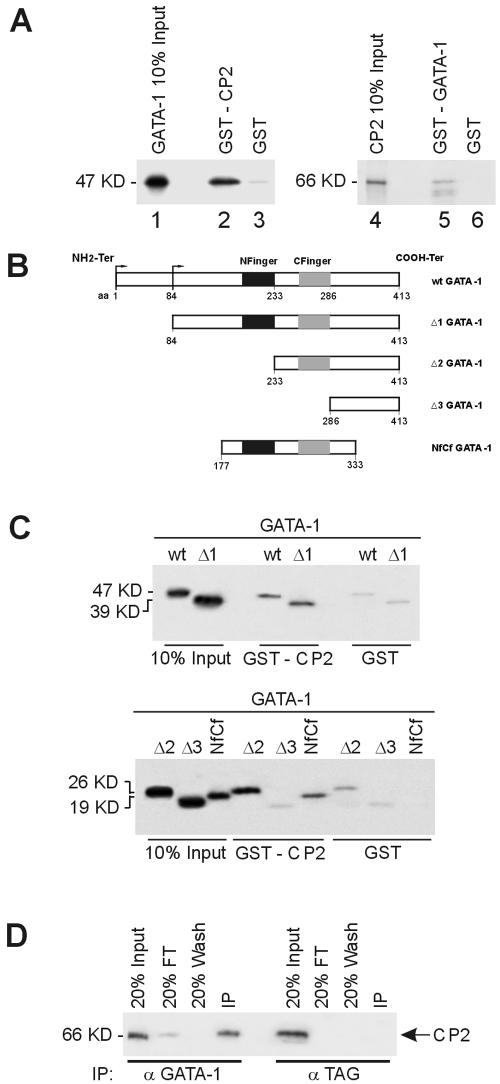
FIG. 7.
Direct physical interaction between GATA-1 and CP2. (A) Purified GST or GST fusion proteins containing full-length CP2 (GST-CP2) preadsorbed to glutathione-Sepharose beads were incubated with 35S-labeled in vitro-transcribed/translated GATA-1 (lanes 1 to 3). Specifically bound protein was eluted from washed beads and visualized by autoradiography after SDS-PAGE. Input represents 10% of the in vitro-translated GATA-1 used in the assay. Lanes 4 to 6 utilized glutathione-Sepharose-bound GST-GATA-1 and 35S-labeled CP2. (B) Schematic of truncation mutants used to map the GATA-1 regions responsible for the interaction with CP2. (C) GST or GST-CP2 protein coupled to glutathione-Sepharose was incubated with 35S-labeled wild type or truncated GATA-1 mutants shown in panel A. Input represents 10% of the in vitro-translated GATA-1 or mutant protein used in the assay. The molecular masses of the proteins are shown. (D) K562 cell extract was immunoprecipitated with either anti-GATA-1 or an unrelated antibody (anti-TAG) as the control. Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with anti-CP2 antibody. CP2 protein was detected in precipitates from anti-GATA-1 antibody but not from the control.