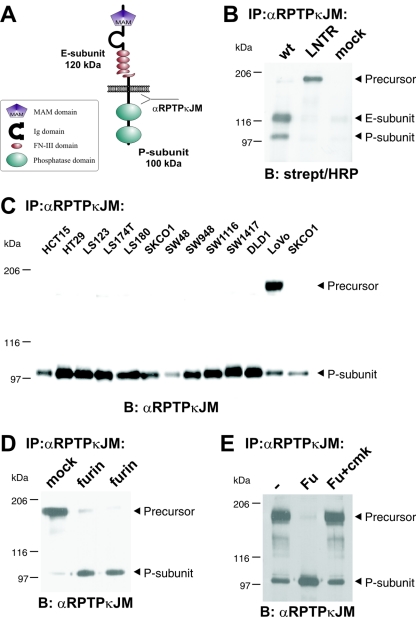
FIG. 1.
RPTPκ is a two-subunit receptor at the cell surface after furin-mediated processing. (A) Scheme depicting the two-subunit structure of RPTPκ. The fragment sizes are indicated. Ig, immunoglobulin. (B) Cell surface-presented RPTPκ is a two-subunit enzyme, composed of the E subunit and the PTP domain-containing P subunit. 293 cells were transfected with wild-type RPTPκ and the convertase cleavage site mutant RPTPκ-LNTR, in which the dibasic sequence motif RTKR located in the membrane-proximal fibronectin type III domain was replaced by LNTR. Cells were surface biotinylated prior to lysis under standard conditions as described in Materials and Methods. RPTPκ was immunoprecipitated by an antibody to the intracellular juxtamembrane part (anti-RPTPκJM), followed by blotting with horseradish peroxidase (HRP)-conjugated streptavidin. (C) Accumulation of the precursor in LoVo cells that are devoid of functional furin. RPTPκ protein was analyzed in a panel of colon carcinoma cell lines by immunoprecipitation and blotting with anti-RPTPκJM antibody. (D) Stable expression of furin in LoVo cells restores processing of RPTPκ. The phosphatase was immunoprecipitated from two LoVo cell clones stably expressing human furin and from a vector-transfected clone (mock) for comparison. (E) Purified furin cleaves RPTPκ within the membrane-proximal fibronectin type III domain at the sequence RTKR in vitro. Furin-null, LoVo cell-derived RPTPκ was immunoprecipitated and incubated for 1 h at 37°C with phosphate-buffered saline (PBS) (−), purified recombinant mouse furin (Fu) or purified recombinant furin previously treated with the inhibitor decRVKR-cmk (Fu+cmk). Abbreviations: α, anti; B, blotting; IP, immunoprecipitation.