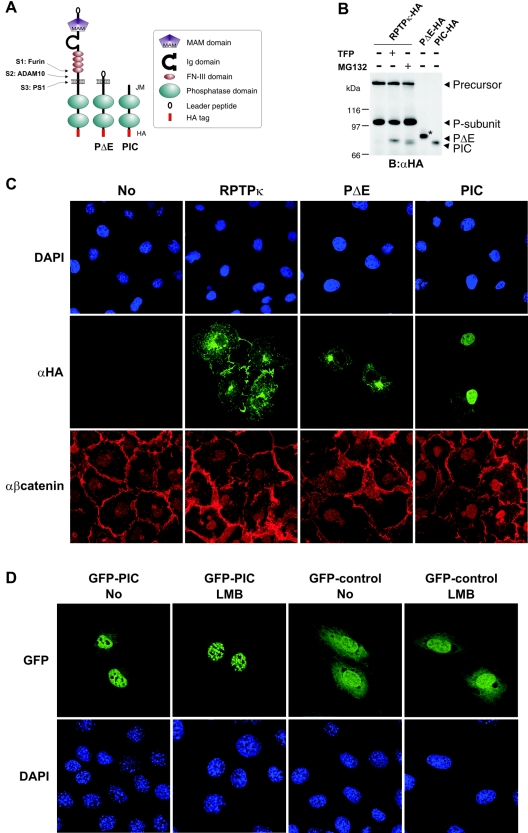
FIG. 5.
Exogenously expressed PIC localizes to the nucleus. (A) Schematic representation of ADAM-derived, transmembrane PΔE- and PS1-derived soluble PIC. All constructs used in this study were HA tagged at the C terminus. Ig, immunoglobulin. (B) Comparison of PS1-derived and recombinant PIC starting at residue 773 at the membrane-cytoplasm interface. COS-7 cells were transfected with HA-tagged RPTPκ and treated with (+) or without (−) TFP or MG132 to induce PΔE and PIC generation. Additionally, recombinant PΔE and PIC constructs were expressed in these cells. For comparison, only 1/5 of PΔE and 1/10 of PIC were transfected. The increase in size of recombinant PΔE (marked by an asterisk) is due to glycosylation (data not shown). B, blotting; α, anti. (C) COS-7 cells were transfected with the indicated HA-tagged RPTPκ isoforms, fixed and immunostained by using anti-HA antibody and AlexaFluor 488-labeled secondary antibody (green). Endogenous β-catenin was detected with a polyclonal antibody to β-catenin and AlexaFluor 546-labeled secondary antibody (red). Nuclear staining with DAPI (4′,6′-diamidino-2-phenylindole) is shown in the upper panels. Note the detection of PIC in the nucleus by confocal microscopy. Due to overexpression, transmembrane PΔE localizes predominantly in the endoplasmic reticulum and Golgi complex. α, anti. (D) Accumulation of PIC in nuclear bodies after the inhibition of CRM1-dependent nuclear export. NIH 3T3 cells were transfected with either GFP-PIC (left panels) or GFP control vector (right panels) and treated or not treated with leptomycin B (LMB) at 25 ng/ml for 3 h. After fixation, cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) and observed with a Leica confocal microscope. Note the specific accumulation of GFP-PIC in nuclear bodies upon leptomycin B treatment.