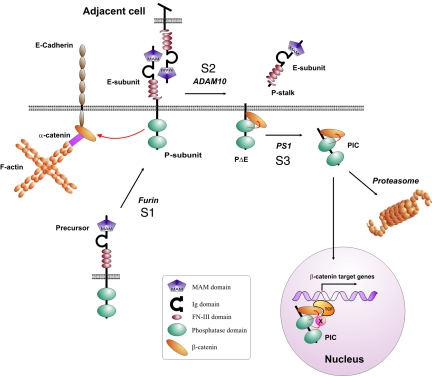
FIG. 8.
Hypothetical model of the RPTP S1/S2/S3 cleavage mechanism and activation of β-catenin-mediated transcription. Furin-mediated cleavage constitutively yields two subunit RPTPκ proteins at the cell surface. Homophilic binding between RPTPκ proteins expressed in trans at high cell densities may induce S2 cleavage by ADAM 10, resulting in the release of the homophilic binding site into the cell supernatant. The remaining part, PΔE, is subject to γ-secretase/PS1-dependent intramembrane proteolysis that allows translocation of catalytically active PIC to the nucleus. Proteasomal destruction decreases the level of PIC in cells and may prohibit its nuclear import. PIC dephosphorylates the coactivator β-catenin and, unlike RPTPκ, increases TCF-mediated transcription. Note that PIC may additionally dephosphorylate transcriptional regulatory proteins associated with the β-catenin/TCF complex. Ig, immunoglobulin.