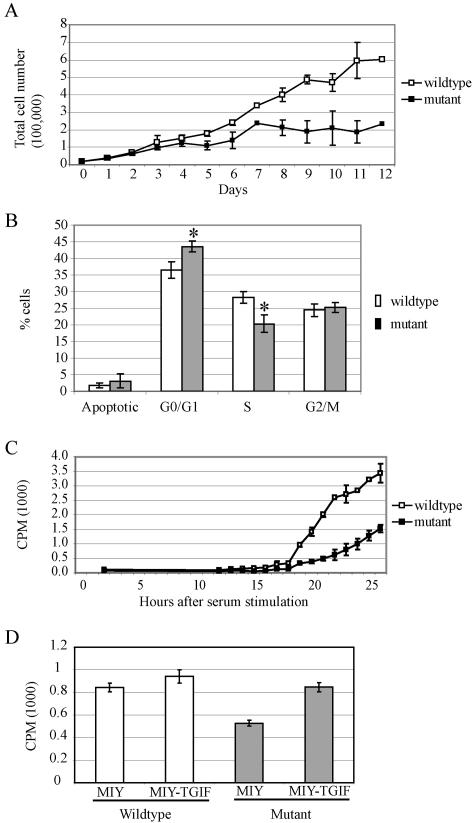
FIG. 3.
Tgif−/− MEFs exhibited reduced growth due to a delay in G1 phase progression. (A) Equal numbers of MEFs were plated and counted daily for 12 days. Tgif−/− cells proliferated at a lower rate and accumulated to a lower density than Tgif+/+ cells. Data are pooled from two experiments using independently derived MEFs. (B) BrdU labeling and FACS analysis indicated Tgif−/− cells accumulated in G0/G1 phase and showed delayed entry into S phase. P values were equal to 0.003 and 0.002 between Tgif+/+ (n = 8) and Tgif−/− (n = 8) cells with regard to the percentage of cells in G0/G1 and S phase, respectively, using single-factor analysis of variance. A representative result is shown from five experiments using independently derived MEFs. (C) Tgif+/+ and Tgif−/− cells were growth arrested by serum starvation, and then serum was added to stimulate synchronous reentry into the cell cycle. DNA synthesis was measured by [3H]thymidine incorporation as described in the text. Fewer mutant MEFs reentered the cell cycle at all time points. Consequently, the total number of mutant MEFs entering into the cell cycle was lower. Representative results from two experiments are shown, each performed in triplicate, using cells derived from independent MEFs. (D) Tgif+/+ and Tgif−/− MEFs were infected with MSCV-retroviruses expressing human TGIF and YFP (MIY-TGIF) or YFP alone (MIY). Infected cells were enriched by FACS, and DNA synthesis was measured by [3H]thymidine incorporation as described in the text. Expression of human TGIF restored the proliferation rate of mutant MEFs to normal levels. Error bars indicate standard deviations. CPM, counts per minute. Data shown here were obtained with MEFs derived from embryos on the 129/Sv/CD1 genetic background.