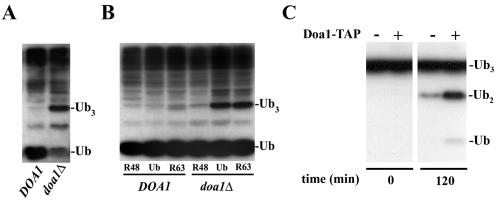
FIG. 3.
Doa1 is required to process K48-linked polyubiquitin trimers (Ub3) into free Ub. (A) The doa1Δ mutant exhibited low levels of free Ub and accumulation of Ub3. Cell lysates derived from the DOA1 (W1588-4C) and doa1Δ (FR657) strains were subjected to anti-Ub Western blotting. (B) Ub3 is K48 linked. Expression of K48R Ub, but not K63R Ub, suppressed the accumulation of Ub3. The DOA1 (W1588-4C) and doa1Δ (FR657) strains were transformed with plasmids expressing Ub (YEp96), K48R Ub (YEp110), or K63R Ub (pTER103). Whole-cell lysates were prepared from mid-log-phase cells treated with CuSO4 (100 μM) for 2 h and subjected to anti-Ub Western blotting. (C) The Doa1 protein complex possesses in vitro Ub protease activity. Cell lysates derived from the wild-type (BY4741) and Doa1-TAP strains were incubated with IgG-Sepharose at 4°C for 5 h. After being washed extensively, beads were resuspended and incubated with K48-Ub3 at 30°C. Proteins were resolved by SDS-PAGE and subjected to anti-Ub Western blotting.