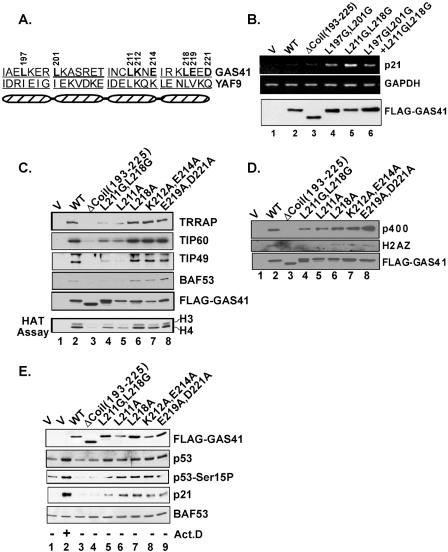
FIG. 3.
Structure-function analysis of the GAS41 coiled-coil motif. (A) Schematic diagram of four heptad repeats in the coiled-coil domain in the GAS41 and YAF9 C termini. The mutated amino acid residues are indicated by position numbers. (B) Disruption of the coiled-coil structure induces p21 gene activation. U2OS cells were transiently transfected with an empty vector (V) or a vector expressing wild-type (WT) or mutant GAS41. At 24 h posttransfection, total RNA and whole-cell extracts were analyzed by RT-PCR and immunoblotting, respectively. (C) Requirement of the GAS41 coiled-coil structure for interaction with the TIP60 complex. 293T cells were transfected with an empty vector or a vector expressing FLAG-GAS41, and whole-cell lysates were immunoprecipitated with M2 agarose beads. Eluates were analyzed by immunoblotting and HAT assays as indicated. (D) Requirement of the GAS41 coiled-coil structure for interaction with p400 and H2AZ. An experiment equivalent to that used for panel C was conducted to analyze interactions of GAS41 mutants with p400 and H2AZ by coimmunoprecipitation. (E) Activation of the p53 tumor suppressor pathway both by GAS41 mutants that are defective in TIP60 and SRCAP complex assembly and by GAS41 mutants that are not. U2OS cells were transiently transfected with the same set of GAS41 mutant vectors as those shown in panel C, and whole-cell lysates were analyzed by immunoblotting as indicated. Cells transfected with an empty vector followed by actinomycin D treatment (10 ng/ml) for 6 h were used as a positive control for activation of the p53 pathway. An immunoblot against BAF53 was used as a loading control.