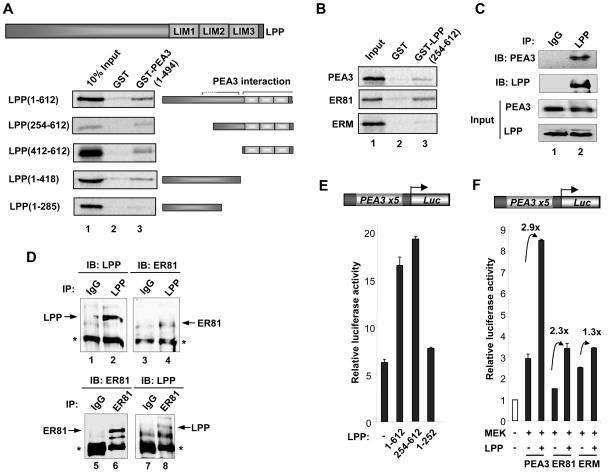
FIG. 6.
Mapping the PEA3 binding surface(s) on LPP. (A) GST pull-downs using GST or GST-PEA3 proteins and in vitro translated LPP derivatives. A diagrammatic representation of full-length LPP is shown at the top, and truncated derivatives of this are depicted next to appropriate lanes. (B) GST pull-downs of GST or GST-LPP(254-612) and in vitro translated zebra fish PEA3, human ER81, and human ERM. Ten percent input protein is shown. (C) Coimmunoprecipitation of LPP and PEA3. 293 cells were transfected with a PEA3 expression vector and immunoprecipitations (IP) were carried out with control IgG or LPP antibodies. Immunoprecipitated LPP and PEA3 were detected by immunoblotting (IB). Inputs show equal amounts of LPP and PEA3. (D) Coimmunoprecipitation of endogenous LPP and ER81 from MDA-MB-231 cells. Immunoprecipitations (IPs) were carried using anti-LPP antibody (top panel) or anti-ER81 antibody (bottom panel), and precipitated LPP and ER81 were detected by immunoblotting (IB) with the appropriate antibodies. (E and F) Reporter gene analysis of the PEA3 site-driven luciferase reporter construct in 293 cells. (E) Mouse PEA3 (200 ng) and the indicated Gal4-LPP constructs (2 μg) were cotransfected. (F) Mouse PEA3, human ER81, or human ERM (200 ng) and constitutively active MEK and LPP (2 μg) were cotransfected. The increase in activation (n-fold) by LPP is indicated above each set of bars.