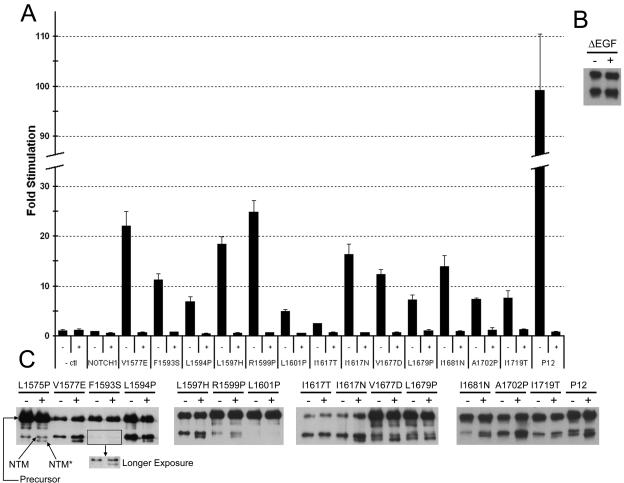
FIG. 4.
HD domain mutations cause increased S2 and S3 cleavage. (A) Gamma secretase inhibitor (GSI) treatment abrogates the stimulatory effects of HD domain mutations in full-length NOTCH1. Immediately following transfection of U2OS cells with NOTCH1 expression plasmids encoding the indicated mutations, cells were treated with 1 μM compound E (GSI) or carrier alone. Trancriptional activation assays were performed 44 to 48 h after transfection, as described in the legend to Fig. 3. The increased signaling produced by the L1575P mutation was also inhibited by GSI in other experiments (data not shown). (B) U2OS cells transfected with wild-type ΔEGF-MYC NOTCH polypeptides were incubated in the presence of 1 μM compound E (GSI; +) or carrier (−) for 18 h prior to harvesting. NTM and NTM* polypeptides were then recovered by preparing immunoprecipitates from cell lysates using anti-MYC-coupled beads, resolved by SDS-PAGE under reducing conditions, and detected by Western blotting using an antibody against intracellular NOTCH1. (C) HD domain mutations were introduced into ΔEGF-MYC polypeptides, and the same assay was performed. Precursor, uncleaved NOTCH1 polypeptide; NTM, transmembrane subunit created by furin cleavage at site S1; and NTM*, S2 cleavage product.