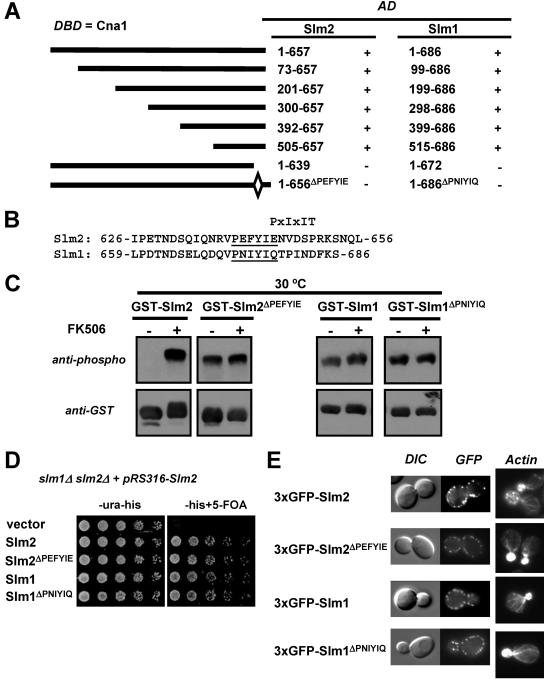
FIG. 2.
Calcineurin interacts with Slm1 and Slm2 via a C-terminal PXIXIT-related motif. (A) Mapping of the calcineurin-docking site on Slm1 and Slm2 via yeast two-hybrid assays. The PJ69-4A strain containing the GAL4 DNA-binding domain fusion of CNA1 (DBD) was transformed with different truncations or internal deletions of SLM1 and SLM2. Positive interactions were identified as growth on synthetic medium lacking histidine (3 days at 30°C). GAL4 activation domain fusions (AD) that interacted with Cna1 are indicated as “+”, and those that did not interact are indicated as “−.” (B) Relevant parts of the amino acid sequences of Slm2 and Slm1 with the conserved calcineurin-docking sites, PXIXIT-related motifs, underlined. (C) Deletion of the calcineurin-docking site abolishes the calcineurin-dependent dephosphorylation of Slm1 and Slm2. Wild-type GST-SLM2 (GBY115) and GST-SLM1 (GBY117) and their counterparts lacking the calcineurin-docking site, GST-SLM2ΔPEFYIE (GBY116) and GST-SLM1ΔPNIYIQ (GBY118), were expressed in a strain deleted for slm1Δ and slm2Δ. Cells were grown in selective synthetic medium supplemented with 4% raffinose. One culture was treated with FK506 (as indicated). GST fusion proteins were purified from cell extracts, subjected to SDS-PAGE, and Western blotted with anti-phospho and anti-GST antibodies. (D) Deletion of the calcineurin-docking site in SLM1 or SLM2 does not impair growth. An slm1Δ slm2Δ strain expressing SLM2 under the control of its own promoter on a pRS316 plasmid (GBY059) was transformed with either pRS313 (vector; GBY152), pRS313-SLM2 (Slm2; GBY153), pRS313-SLM2ΔPEFYIE (Slm2 ΔPEFYIE; GBY154), pRS313-SLM1 (Slm1; GBY155), or pRS313-SLM1ΔPNIYIQ (Slm1ΔPNIYIQ; GBY156). Serial dilutions of saturated cell cultures were plated on synthetic medium lacking uracil and histidine (-ura-his) and on synthetic medium lacking histidine but supplemented with 5FOA (-his+5-FOA). Cells were grown for 3 days at 30°C. (E) Localization of Slm proteins and actin cytoskeleton stabilization by Slm proteins are not dependent on the calcineurin-docking site. slm1Δ slm2Δ cells expressing GFP-SLM2 (GBY104), GFP-SLM2ΔPEFYIE (GBY105), GFP-SLM1 (GBY106), or GFP-SLM1ΔPNIYIQ (GBY107) were grown to log phase and visualized by differential interference microscopy (DIC) and by fluorescence microscopy (GFP). Actin cytoskeleton was visualized with Texas Red-X phalloidin in formaldehyde-fixed cells.