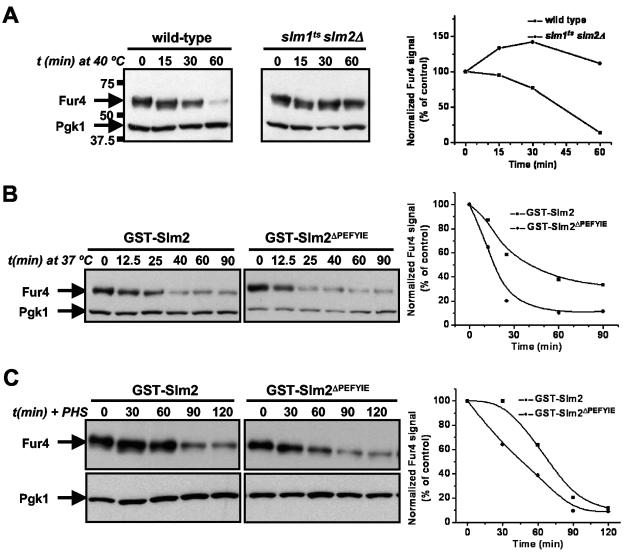
FIG. 9.
Slm function is required for heat shock-induced Fur4 turnover. (A) Wild-type cells (SEY6210.1) and slm1ts slm2Δ cells (AAY1623.2) carrying pVTu-FUR4 (GBY179 and GBY180, respectively) were shifted from 25°C to 40°C and samples were taken at time points (t) just prior to (0 min) and 15, 30, and 60 min after heat shock. Cell lysates were analyzed by SDS-PAGE and immunoblotting with anti-Fur4 and anti-PGK antibodies. Pgk1 was used as a loading control, which also shows the absence of nonspecific protein degradation by heat shock. The numbers to the left of the gels indicate the molecular masses of the protein standards. Immunoreactive bands were quantified as described in Materials and Methods. The ratio of the anti-Fur4 signal to the anti-Pgk1p signal is shown as a function of time. The value at 0 min was used as the reference value and set at 100%. (B) slm1Δ slm2Δ cells expressing either GST-SLM2 or GST-SLM2ΔPEFYIE and overexpressing FUR4 (GBY115 and GBY116, respectively) were shifted from 25°C to 40°C and sampled at the indicated time points. Cell lysates were analyzed by immunoblotting and quantified as described for panel A. (C) GBY115 and GBY116 were grown at 30°C, incubated with 20 μM phytosphingosine, and sampled at the indicated time points. Lysates were analyzed and signals quantified as described for panel A.