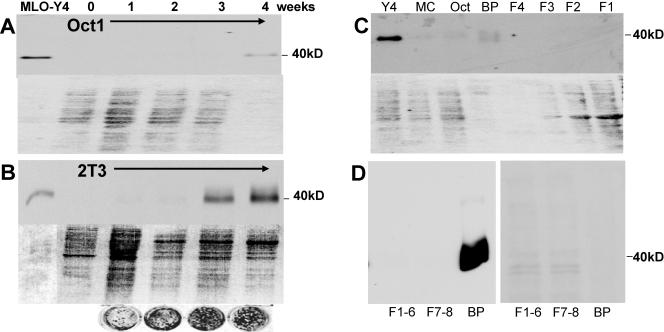
FIG. 1.
Identification of a 40-kDa protein highly expressed in osteocytes. With monoclonal antibody 9C11, made against MLO-Y4 cells, a 40-kDa band was identified by Western blotting and shown to be highly expressed in MLO-Y4 osteocyte-like cells. The top part of each panel is a Western blot assay, and the bottom is a Ponceau S-stained gel to show relative amounts of loaded protein. Theoretically, as osteoblasts form a mineralized matrix, any cells trapped in that matrix would have the characteristics of osteocytes. Under mineralizing culture conditions, the Oct-1 cells began to express this antigen at 4 weeks (A) and the 2T3 cells began to express this antigen earlier and in large amounts, at 3 and 4 weeks of culture (B). The circles are von Kossa-stained 2T3 cultures showing increased mineralization with time. This suggests that these cells are differentiating into osteocytes in culture. The 9C11 antibody also recognized a band in bone extracts (C). The 40-kDa band is present in MLO-Y4 cells but was not present in osteoblast-like cells such as MC3T3 (MC) and Oct-1 cells (Oct), nor was it visible in extracted cells. These cells were isolated from 6-week-old mouse long bone through serial digestions of collagenase with and without EDTA (F1, F2, F3, and F4). Only the bone particles (BP) containing embedded osteocytes showed the 40-kDa band. Note the relative amounts of protein in the particle fraction compared to protein in the cell fractions. Similar experiments were repeated with the 8.1.1 antibody (D). The Western blot assay is on the left, and the Ponceau stain is on the right. F1 to F6 represent cells on the bone surface that are removed by serial digestions with collagenase. F7 and F8 represent cells on the bone surface that could be removed by EDTA, followed by collagenase. Bone particles (BP) represent the remaining bone that was subjected to boiling in SDS sample buffer. Note that considerably less protein was loaded in the well containing the bone particle extracted protein.