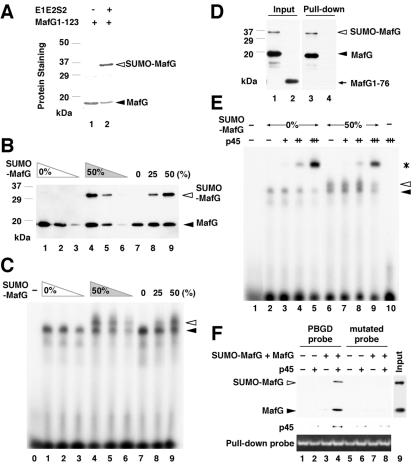
FIG. 3.
Analysis of DNA binding and dimerization of sumoylated MafG. (A) SUMO conjugation to His-MafG 1-123 in E. coli. Total lysates from bacteria harboring pET15b-MafG1-123 (lane 1) and pET15b-MafG1-123 plus pT-E1E2S2 (lane 2) were incubated with nickel beads. The associated proteins were analyzed by SDS-PAGE followed by Coomassie blue staining. (B and C) DNA binding ability of bacterially synthesized His-MafG 1-123 and its sumoylated form. EMSA was performed with a MARE-containing probe (C). The incubated proteins are shown in the immunoblot reacted with anti-His antibody (B). The lane numbers in panel B correspond to those in panel C. No protein was added to the reaction mixture in lane 0 in panel C. (D) Heterodimer formation between sumoylated or nonsumoylated His-MafG 1-123 and MBP-p45. The mixture of sumoylated and nonsumoylated His-MafG 1-123 was incubated with MBP-p45 (lanes 1 and 3). His-MafG 1-76 was incubated with MBP-p45 as a control (lanes 2 and 4). Input proteins and pull-down samples were reacted with anti-His antibody. (E) DNA binding ability of His-MafG 1-123 or its sumoylated form in the presence of MBP-p45. Constant amounts of His-MafG 1-123 (lanes 2 to 5) and the mixture of His-MafG 1-123 and its sumoylated form (lanes 6 to 9) were incubated with a MARE-containing probe and increasing amounts of MBP-p45. No protein was added to the reaction mixture in lane 1, and only MBP-p45 was added to lane 10. (F) DNA binding of heterodimers containing MafG and p45. The mixture of sumoylated and nonsumoylated His-MafG 1-123 (lane 9) and/or MBP-p45 was incubated with biotinylated PBGD probe (lanes 1 to 4) or a control mutated probe (lanes 5 to 8). A protein interaction was observed only when both the MafG mixture and MBP-p45 were added to the PBGD probe (lane 4). The pull-down efficiency was monitored by the quantity of DNA probe interacting with the avidin resins. The percentages shown at the tops of the panels indicate the approximate molar ratios of sumoylated His-MafG 1-123 to the total of sumoylated and nonsumoylated His-MafG 1-123 used in the reactions (B, C, and E). White and black arrowheads indicate His-MafG 1-123 with or without sumoylation, respectively (A, B, and D), or a shifted band generated by His-MafG 1-123 and the one containing sumoylated His-MafG 1-123, respectively (C and E). An asterisk indicates a shifted band representing heterodimer binding (E).