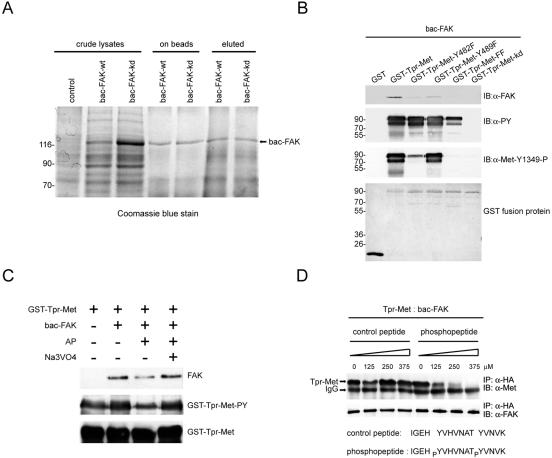
FIG. 3.
Direct interaction between Met and FAK. (A) Baculovirus-expressed recombinant FAK proteins (bac-FAK) were purified from insect cell lysates by affinity chromatography. The crude insect cell lysates, the protein A-Sepharose-bound proteins (on beads), and the eluted proteins (eluted) were fractionated by SDS-polyacrylamide gel electrophoresis and visualized by Coomassie blue staining. (B) Immobilized GST fusion proteins encoding Tpr-Met or its mutants were incubated with purified baculovirus-expressed FAK proteins, and the bound proteins were analyzed by immunoblotting (IB) with anti-FAK (α-FAK). Total tyrosine phosphorylation of GST-Tpr-Met and its specific phosphorylation at Tyr-482 (equivalent to Tyr-1349 of c-Met) were analyzed by immunoblotting with antiphosphotyrosine (α-PY) and anti-Met-Y1349-P, respectively. The GST fusion proteins on the nitrocellulose membrane were visualized by staining with Ponceau S solution. (C) Dephosphorylation of Tpr-Met decreases its association with FAK in vitro. Immobilized GST-Tpr-Met was treated with 10 U of alkaline phosphatase (AP) in the presence or absence of 2 mM sodium vanadate (Na3VO4) for 1 h and then incubated with baculovirus-expressed recombinant FAK. The bound recombinant FAK proteins were analyzed by immunoblotting with anti-FAK. The tyrosine phosphorylation of GST-Tpr-Met was analyzed by immunoblotting with anti-PY. (D) HEK 293 cell lysates containing Tpr-Met were incubated with baculovirus-expressed recombinant FAK proteins in the presence of different amounts of the phosphopeptide or its unphosphorylated counterpart. One hour later, coprecipitation of Tpr-Met and baculovirus-expressed recombinant FAK was analyzed. Molecular size markers (in kilodaltons) are noted at the left of blots. IP, immunoprecipitation; IgG, immunoglobulin G.