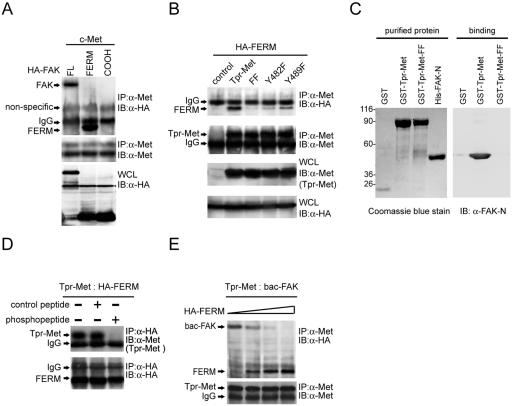
FIG. 4.
The FERM domain of FAK binds to Met. (A) HA-tagged full-length (FL) FAK or its FERM domain (aa 30 to 377) or COOH domain (aa 693 to 1053) was transiently coexpressed with c-Met in HEK 293 cells. Coimmunoprecipitation (IP) of HA-tagged FAK proteins and c-Met was analyzed. (B) The HA-tagged FAK FERM domain was transiently coexpressed with Tpr-Met or its mutants in HEK 293 cells, and their coprecipitation was analyzed. (C) Purified GST-Tpr-Met and the histidine-tagged NH2 domain of FAK (His-FAK-N) were analyzed by SDS-polyacrylamide gel electrophoresis and visualized by Coomassie blue staining (left). Soluble GST-Tpr-Met or its FF mutant was incubated with purified His-FAK-N. One hour later, glutathione agarose beads were added to the mixture and the bound proteins were analyzed by immunoblotting (IB) with an anti-FAK antibody (A-17) specific to the NH2 domain of FAK (α-FAK-N) (right). Molecular size markers (in kilodaltons) are noted at the left of blots. (D) HEK 293 cell lysates containing Tpr-Met and those containing the HA-tagged FAK FERM domain were mixed and incubated with (+) or without (−) 200 μM of the phosphopeptide IGEHPY1349VHVNATPY1356VNVK or its unphosphorylated peptide. One hour later, coprecipitation of Tpr-Met and the HA-tagged FAK FERM domain was analyzed. (E) The FAK FERM domain competes with the full-length FAK for Tpr-Met binding in vitro. HEK 293 cell lysates containing Tpr-Met were incubated with baculovirus-expressed FAK (bac-FAK) in the presence of increasing amounts of HEK 293 cell lysates containing the HA-tagged FAK FERM domain. Coprecipitation of Tpr-Met and HA-tagged proteins was analyzed. IgG, immunoglobulin G; WCL, whole-cell lysates.