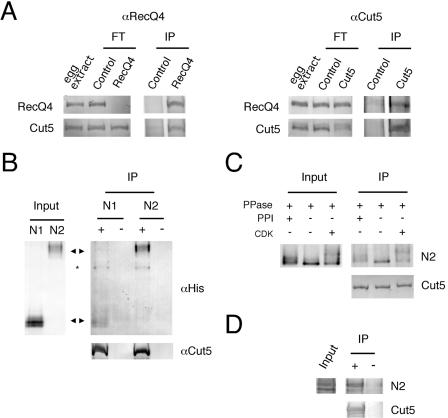
FIG. 8.
Physical interaction between RecQ4 and Cut5. (A) Coimmunoprecipitation of RecQ4 and Cut5. The egg extracts (80 μl) were incubated with preimmune control immunoglobulin G (Control), anti-RecQ4 (RecQ4), or anti-Cut5 (Cut5) antibody-conjugated protein A beads. The untreated 1 μl of egg extract, flowthrough extracts (FT), and the proteins bound to the beads (IP) were resolved by SDS-PAGE and blotted onto nitrocellulose membrane for immunostaining. (B) Physical interaction of RecQ4 and Cut5 in vitro. Recombinant N1- and N2-RecQ4 fragments were incubated with anti-Cut5 antibody (−) or the antibody prebound with Cut5 (+) conjugated to protein A beads, and proteins bound to the antibody were pulled down with the beads. Input fragments (Input) and immunoprecipitated fragments (IP) were resolved by SDS-PAGE and blotted onto a nitrocellulose membrane for immunostaining. Arrowheads indicate the position of each fragment. *, degradation product of Cut5. (C) Effect of phosphorylation of RecQ4 on the interaction with Cut5. Recombinant N2-RecQ4 fragment was treated with 5.6 μg/ml protein phosphatase 1 with (+) or without (−) phosphatase inhibitor (1 μM okadaic acid). For rephosphorylation, the sample treated with the phosphatase was further incubated with 17 μg/ml cyclin A/Cdk2 in the presence of 1 μM okadaic acid to prevent the dephosphorylation. The samples were then incubated with Cut5 prebound to anti-Cut5 antibody. Both input (Input) and immunoprecipitated N2 fragments (IP) were resolved by SDS-PAGE and blotted onto a nitrocellulose membrane for immunostaining. (D) Physical interaction of unphosphorylated RecQ4 and Cut5 in vitro. The experiment was carried out as described for panel B except that the N2 fragment expressed in E. coli was used instead of the protein expressed in Sf9 cells.