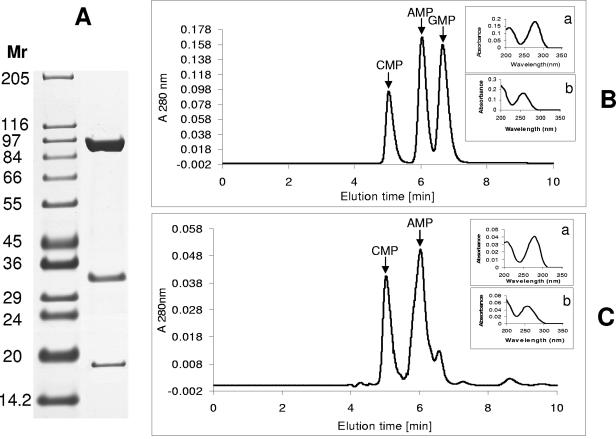
FIG. 1.
Purification of KDH and determination of CMP as a component of its molybdenum dinucleotide cofactor. (A) His-tagged KdhL was used to isolate the KDH holoenzyme by Ni-Sepharose chelation from extracts of bacteria transformed with pART2kdhL. Shown are the three protein subunits of KDH revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (B) HPLC elution profile of CMP, AMP, and GMP standard. (C) KDH was treated as described previously (18), and the released mononucleotides were analyzed by HPLC. CMP and AMP peaks are indicated by arrows. The insets in panels B and C show the UV spectra of CMP, with an absorption peak at 280 nm (a), and of AMP and GMP, with an absorption peak at 260 nm (b).