Abstract
We have evaluated the diagnostic utility of six antigenic regions of the Toxoplasma gondii MIC2, MIC3, M2AP, GRA3, GRA7, and SAG1 gene products, assembled in recombinant chimeric antigens by genetic engineering, in order to replace the soluble, whole-cell tachyzoite extract in serological assays. Serum samples from 100 adults with acquired T. gondii infection and from 30 infants born to mothers with primary toxoplasmosis contracted during pregnancy, of whom 20 were congenitally infected, were included. Immunoglobulin G (IgG) and IgM antibodies against epitopes carried by chimeric antigens were measured by performing parallel enzyme immunoassays (recombinant enzyme-linked immunosorbent assays [Rec-ELISAs]), and the results obtained by standard commercial assays with the whole-cell Toxoplasma antigen and assays with the chimeric antigens were compared. Our results demonstrate that IgG and IgM Rec-ELISAs with individual chimeric antigens have performance characteristics comparable to those of the corresponding commercial assays. Furthermore, we show that IgM-capture assays based on chimeric antigens improve the ability to diagnose congenital toxoplasmosis postnatally compared with the ability to diagnose congenital toxoplasmosis by the use of standard assays. The use of recombinant chimeric antigens is effective in distinguishing T. gondii-infected individuals from T. gondii-uninfected individuals and shows that immunoassays based on recombinant products could provide the basis for standardized commercial tests for the serodiagnosis of toxoplasmosis.
Human infection by Toxoplasma gondii is generally asymptomatic and induces a self-limiting disease. In contrast, primary T. gondii infection acquired during gestation can be transmitted to the fetus through the placenta and may cause miscarriage, permanent neurological damage, premature birth, and visual impairment (15, 29, 32). Toxoplasmosis during gestation represents a formidable task for the clinician due to its subclinical course in the majority of pregnant women and the unpredictable long-term outcome of congenital infection (11, 16, 32). To implement suitable therapies in good time and to avoid neonatal malformations or reduced eyesight in newborns, it is essential to establish when the primary infection has been acquired in the mother and to determine if vertical transmission to the fetus has occurred.
The diagnosis of T. gondii infection can be established by detecting parasite-specific DNA sequences in body fluids and tissues or Toxoplasma-specific immunoglobulins in sera from infected individuals (2, 20, 30). Serological methods are generally preferred, since the sensitivities and the specificities of other methods can be affected by the appropriateness of sample handling and shipping and storage conditions. Furthermore, parasitemia may be of short duration, further limiting the value of detection of DNA in amniotic fluid and peripheral blood.
The diagnosis of toxoplasmosis by serological methods routinely uses immunoenzymatic assays to detect the presence of the various classes of anti-Toxoplasma immunoglobulins (immunoglobulin G [IgG], IgM, and IgA) (29). Most of the commercially available assays use the whole T. gondii soluble extract as the antigen. However, the assays currently available for the detection of specific anti-Toxoplasma antibodies may vary in their abilities to detect serum immunoglobulins (18, 23, 24, 34), due to the lack of a purified standardized Toxoplasma antigen or standard methods for preparation of the antigen. Moreover, in 30% to 60% of infants with congenital toxoplasmosis, the Toxoplasma-specific IgM antibody response is absent or undetectable by standard serological assays (7, 8, 10, 13). Therefore, the availability of innovative diagnostic methods would be desirable.
During the last 10 years, several studies have reported on the use of recombinant antigens for the serological diagnosis of T. gondii infection (1, 4, 8, 17, 21, 22, 27, 31). Nevertheless, although they are promising, none of the assays based on recombinant antigens displayed all the characteristics required to replace the tachyzoite antigen in IgG- and IgM-based tests, indicating that further work is needed before an immunoassay with recombinant products will be available for clinical purposes.
The aim of this study was to improve the performance of enzyme-linked immunosorbent assays (ELISAs) based on recombinant products (Rec-ELISAs).
MATERIALS AND METHODS
Cloning of chimeric antigens.
The DNA encoding the T. gondii MIC2 (residues 157 to 235) (33), MIC3 (residues 234 to 307) (14), and SAG1 (residues 182 to 312) (9) antigens was amplified from T. gondii cDNA (RH strain) by PCR with oligonucleotides K551 and K553, K552 and K555, and K554 and K556, respectively. The PCR products of MIC2 and MIC3 were mixed and then used as templates in a PCR with oligonucleotides K551 and K555 (20 cycles; 30 s at 94°C, 30 s at 50°C, and 60 s at 72°C). The resulting DNA was purified and mixed with the PCR product of SAG1, and the mixture was used as the template for DNA amplification with primers K551 and K556 (20 cycles of 30 s at 94°C, 30 s at 50°C, and 90 s at 72°C) to generate the chimeric antigen EC2. The DNA sequences encoding GRA3 (residues 36 to 134) (6), GRA7 (residues 24 to 102) (12), and M2AP (residues 37 to 263) (28) were amplified with oligonucleotides K563 and K565, K564 and K567, and K566 and K568, respectively. The DNA products of GRA3 and GRA7 were mixed and then used as templates in a PCR with oligonucleotides K563 and K567 (20 cycles of 30 s at 94°C, 30 s at 45°C, and 60 s at 72°C). The resulting DNA was purified and mixed with the PCR product of M2AP, and the mixture was used as the template for DNA amplification with primers K563 and K568 (30 cycles of 30 s at 94°C, 30 s at 45°C, and 180 s at 72°C) to generate the chimeric antigen EC3. Finally, the DNA products of EC2 and EC3 were digested with the SpeI and NotI restriction endonucleases and subcloned into the bacterial vector pGEX-SN (25).
Table 1 shows the oligonucleotides used for the construction of the recombinant EC2 and EC3 antigens.
TABLE 1.
Oligonucleotide primers used for construction of the EC2 and EC3 antigens
| T. gondii gene | Primer name | Primer sequence |
|---|---|---|
| MIC2 | K551 | 5′-GGACTAGTCGGCTCCCCCAGGATGCC-3′ |
| K553 | 5′-CATCCAGTCCTGCTACCGCCACCAGACCAGACGCCACATCCAGC-3′ | |
| MIC3 | K552 | 5′-GTGGCGTCTGGTCTGGTGGCGGTAGCAGGACTGGATGTCATGCC-3′ |
| K555 | 5′-TGACGACCGAGCTACCGCCACCAGAGTTATCGCATTTGCAGGATG-3′ | |
| SAG1 | K554 | 5′-ATGCGATAACTCTGGTGGCGGTAGCTCGGTCGTCAATAATGTCGC-3′ |
| K556 | 5′-CCGCGGCCGCTAGCCGATTTTGCTGACCCTG-3′ | |
| GRA3 | K563 | 5′-GGACTAGTCGGCTGGCTGCCTTGGGAGGCCTTG-3′ |
| K565 | 5′-GCCGCGGTAGCACTACCGCCACCAGACAAACCAGGGCGATCTGTG-3′ | |
| GRA7 | K564 | 5′-GCCCTGGTTTGTCTGGTGGCGGTAGTGCTACCGCGGCCACCGCG-3′ |
| K567 | 5′-CCGGTTCGTTACTACCGCCACCAGAGAAATGAACTTCTTCTTGTTC-3′ | |
| M2AP | K566 | 5′-GAAGTTCATTTCTCTGGTGGCGGTAGTAACGAACCGGTGGCCCTAG-3′ |
| K568 | 5′-CCGCGGCCGCAGATTCAGACTCAGACGGAC-3′ |
Purification and biochemical modification of recombinant antigens.
Recombinant proteins produced in Escherichia coli as fusion proteins with glutathione S-transferase (GST) were purified from the cytoplasm of bacterial cells by affinity chromatography, as described previously (3, 5). Briefly, recombinant E. coli was induced with isopropyl-β-d-thiogalactopyranoside, centrifuged, and suspended in STE buffer (10 mM Tris-HCl [pH 8], 150 mM NaCl) containing 100 μg/ml of lysozyme and protease inhibitor cocktail (Boehringer, Mannheim, Germany). The mixture was sonicated, and Triton X-100 was added to a final concentration of 1%. After centrifugation at 10,000 × g for 30 min at 4°C, the supernatant was incubated with glutathione-Sepharose (Amersham-Pharmacia Biotech, Sweden), and GST-proteins were eluted by following the manufacturer's instructions. Finally, protein purity and content were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration analysis and by Bradford assays, respectively.
For biotin labeling, the GST-EC2 and GST-EC3 fusion proteins were diluted at a concentration of 1 mg/ml in phosphate-buffered saline (PBS) and then incubated in the presence of a fivefold molar excess of sulfosuccinimidyl-6-(biotin-amido)hexanoate (Sulfo-NHS-LC-Biotin; Pierce) for 3 h on ice. The proteins were then dialyzed against PBS overnight to remove excess nonreacted and hydrolyzed biotin reagents. The levels of biotin incorporation into recombinant antigens were determined by using an EZ Biotin quantitation kit (Pierce), following the instructions of the manufacturer. The biotin-labeled products were diluted to a concentration of 0.5 mg/ml and finally stored at −20°C.
Serum samples. (i) Adults.
One hundred twenty-seven serum samples from 127 women, collected at the Center for Perinatal Infection of the Campania Region of Italy were used. Accordingly to the results obtained by analyzing sera by the standard enzyme-linked fluorescent assays (ELFAs) ELFA-Toxo-IgG and ELFA-Toxo-IgM (bioMerieux, Marcy l'Etoile, France), serum samples were divided into three groups, as follows: group A (n = 50) was composed of samples with a serological profile characterized by the presence of T. gondii-specific IgG and the absence of T. gondii-specific IgM, group B (n = 50) was composed of samples with a serological profile characterized by the presence of T. gondii-specific IgM and IgG antibodies, and group C (n = 27) was composed of samples negative for T. gondii-specific IgG and IgM antibodies.
(ii) Infants.
Thirty serum samples from 30 infants born to mothers with primary toxoplasmosis in pregnancy and referred for postnatal follow-up at the Center for Perinatal Infection were included in the study. The maternal diagnosis of primary T. gondii infection was based on seroconversion during gestation. Serum samples from infants were analyzed for the presence of specific anti-Toxoplasma IgM and IgA in the first 3 months of age and for specific IgG at birth and at 1, 2, 3, 6, 9, and 12 months of age (tested by IgG-specific ELFA [ELFA-IgG], ELFA-IgM, and IgA-specific ELISA [ELISA-IgA]; see below). The diagnosis of congenital toxoplasmosis was proven, retrospectively, on the basis of the persistence of Toxoplasma-specific IgG antibodies after 12 months of age and was excluded by their disappearance. Twenty infants had congenital infection, and 10 were uninfected. Brain untrasonography, cranial computed axial tomography, and indirect fundoscopy established the severity of clinical onset. The disease onset was considered severe, benign, or subclinical accordingly to the criteria of Hohlfeld et al. (19).
Whole-cell Toxoplasma antigen immunoassays.
Analysis of Toxoplasma-specific IgG, IgM, and IgA antibodies and measurement of anti-Toxoplasma IgG titers (given as international units/ml) in serum samples were done by the whole-cell, Toxoplasma antigen assays ELFA-IgG, ELFA-IgM, and IgM-specific immunosorbent agglutination assay (ISAGA-IgM) (bioMerieux); an ELISA-IgA (Sanofi-Pasteur, Marnes La Coquette, France); and the ETI-TOXOK-M reverse PLUS assay (Diasorin, Saluggia, Italy), according to the manufacturers' instructions. All serum samples were analyzed in a blinded fashion.
Recombinant protein enzyme immunoassays. (i) IgG-specific recombinant ELISA (IgG Rec-ELISA).
Maxisorb multiwell plates (Nunc) were adsorbed with recombinant proteins at a concentration of 5 μg/ml in coating buffer (50 mM NaHCO3, pH 9.6). Each recombinant protein (GST-MIC2, GST-MIC3, GST-SAG1, GST-GRA3, GST-GRA7, GST-M2AP, GST-EC2, and GST-EC3) was coated separately onto individual microtiter wells. After incubation overnight at 4°C, the plates were blocked for 1 h at 37°C with 5% nonfat dry milk and 0.05% Tween 20 in PBS (blocking buffer) and were subsequently incubated for 1 h at 37°C with serum samples diluted 1:200 in blocking buffer. The plates were extensively washed with 0.05% Tween 20 in PBS, and anti-human IgG horseradish peroxidase-conjugated antibodies (Sigma-Aldrich) were then added to each well. Finally, incubation of the plates with the chromogenic substrate tetramethylbenzidine (Sigma-Aldrich) revealed the enzymatic activity. The results were recorded as the difference between the absorbance (optical density [OD]) at 450 nm and that at 620 nm, as detected with an automated ELISA reader (Labsystem Multiskan, Finland). For each serum sample, the assay was done in duplicate and average values were calculated. For every GST-fusion product, the cutoff value was determined as the mean plus two times the standard deviation of the absorbance readings obtained for the Toxoplasma IgG-negative sera.
(ii) IgM Rec-ELISA.
Maxisorb plates (Nunc) were coated with anti-human IgM antibodies (kindly provided by Fabrizio Bonelli, Diasorin, Italy) at a concentration of 5 μg/ml in coating buffer. The plates were blocked with 3% bovine serum albumin in PBS (PBS/BSA buffer) for 1 h at 37°C and were subsequently incubated for 1 h at 37°C with serum samples (diluted 1:100) in PBS/BSA buffer. The plates were washed and then incubated for 2 h at room temperature with the biotin-labeled GST-fusion proteins, diluted at 500 ng/ml in PBS/BSA buffer. After the plates were extensively washed, they were incubated for 30 min at room temperature with horseradish peroxidase-conjugated streptavidin (Pierce) at a concentration of 1 μg/ml in PBS/BSA buffer. Finally, the enzymatic activity was revealed by incubating the plates for 30 min at room temperature with tetramethylbenzidine, and the results were recorded as described above. For each serum sample the assay was done in duplicate, and average values were calculated. For each biotin-labeled antigen the cutoff value was determined as the mean plus two times the standard deviation of the absorbance readings obtained for the Toxoplasma-specific IgM-negative sera.
To determine the optimal assay conditions, biotin-labeled GST-EC2 and GST-EC3 were challenged with a serum sample with a high titer of Toxoplasma-specific IgM antibodies. To analyze the thermal stability of the chimeric antigens, the biotin-labeled GST-EC2 and GST-EC3 proteins were stored at 4°C in diluted solutions (250 ng/ml in Stabilzyme buffer [Surmodics]) and assayed by the IgM Rec-ELISA after different time intervals.
(iii) Antibody depletion.
Distinct mixtures of GST-fusion products were coated onto Maxisorb plates at a concentration of 10 μg/ml in coating buffer. The plates were blocked as described above for the IgG Rec-ELISA and then incubated for 30 min at 37°C with serum samples (20 μl/well) in blocking solution (5% nonfat dry milk, 0.05% Tween 20 in PBS). The antigen-specific antibody-depleted sera were recovered from each well, added to a new antigen-coated well, and further incubated for 30 min; and the procedure was repeated five more times. Sera that had been depleted of specific antibodies against combinations of GST-fusion products were finally analyzed by the IgG Rec-ELISAs, as described above.
Statistical analysis.
The sensitivity, specificity, agreement, and positive and negative predictive values of the enzyme-linked immunosorbent assays are given. Proportions were compared by the chi-square test, with P values of <0.05 considered statistically significant.
RESULTS
Expression and characterization of recombinant chimeric antigens.
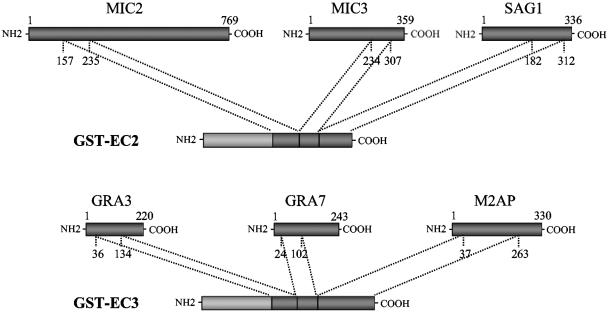
The cDNA fragments encoding the antigenic regions of the Toxoplasma gondii antigens MIC2, MIC3, SAG1, GRA3, GRA7, and M2AP (3, 5) were assembled by PCR to give the chimeric antigens EC2 and EC3 (Fig. 1), which were then cloned into vector pGEX-SN (25). This resulted in the production of GST-fusion proteins and allowed their purification from E. coli cells under native conditions by one-step affinity chromatography. The recombinant chimeric antigens were efficiently expressed and purified in large amounts from the cytoplasm of bacterial cells, with the yields of purified GST-EC2 and GST-EC3 antigens being 8 and 5 mg per liter of bacterial culture, respectively. The results of the gel filtration analysis established that the purified GST-EC2 and GST-EC3 chimeric antigens were homogeneously associated in dimeric molecules with molecular masses of 110.4 kDa and 152.6 kDa, respectively, and with purities of 90 to 95% (data not shown).
FIG. 1.
Schematic representation of chimeric antigens EC2 and EC3. The antigenic regions of the T. gondii MIC2, MIC3, SAG1, GRA3, GRA7, and M2AP gene products that were used to construct the GST-EC2 and GST-EC3 fusion proteins are shown.
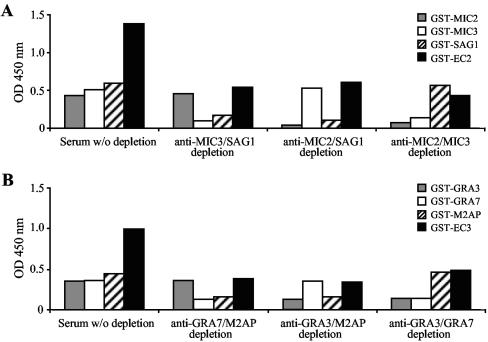
Next, to verify that the immunoreactivities of each of the individual antigenic regions were retained by the chimeric antigens, serum samples characterized by the presence of Toxoplasma-specific IgG antibodies were adsorbed against different combinations of antigen fragments and subsequently assayed with GST-EC2 and GST-EC3 fusion proteins by IgG Rec-ELISAs. As shown in Fig. 2, all six antigen fragments (MIC2, MIC3, SAG1, GRA3, GRA7, and M2AP) assembled into EC2 and EC3 chimeric antigens retained their individual immunoreactivities against specific anti-Toxoplasma IgG antibodies compared to the immunoreactivities of the corresponding antigenic regions analyzed as distinct GST-fusion products.
FIG. 2.
Antigenic properties of individual protein fragments within the EC2 and the EC3 chimeric antigens. The immunoreactivities of GST-MIC2, GST-MIC3, GST-SAG1, and GST-EC2 (A) or GST-GRA3, GST-GRA7, GST-M2AP, and GST-EC3 (B) with IgG antibodies from T. gondii-infected individuals were analyzed by use of whole serum or serum after depletion of antibodies against combinations of antigen fragments (MIC2/MIC3 depletion, MIC2/SAG1 depletion, etc.). w/o, without.
Immunoreactivities of chimeric antigens with IgG and IgM antibodies from sera of T. gondii-infected individuals.
Table 2 shows the performance characteristics of the IgG Rec-ELISAs with the GST-EC2 and GST-EC3 chimeric antigens in comparison to the results obtained with the individual antigen fragments. Each chimeric antigen reacted with 100% of the positive sera, and none of the Toxoplasma-negative sera reacted with the GST-EC2 and GST-EC3 proteins.
TABLE 2.
Diagnostic performance of the IgG immunoassays based on recombinant antigensa
| IgG Rec-ELISA antigen | Pb | Sensitivity (%) | Specificity (%) | Agreement (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| GST-MIC2 | <0.0001 | 92 | 100 | 94.8 | 100 | 87.1 |
| GST-MIC3 | <0.0001 | 90 | 100 | 93.5 | 100 | 84.4 |
| GST-SAG1 | <0.0001 | 82 | 100 | 88.3 | 100 | 75 |
| GST-GRA3 | <0.0001 | 82 | 100 | 88.3 | 100 | 75 |
| GST-GRA7 | <0.0001 | 88 | 100 | 92.2 | 100 | 81.8 |
| GST-M2AP | <0.0001 | 78 | 100 | 85.7 | 100 | 71.1 |
| GST-EC2 | <0.0001 | 100 | 100 | 100 | 100 | 100 |
| GST-EC3 | <0.0001 | 100 | 100 | 100 | 100 | 100 |
PPV, positive predictive value; NPV, negative predictive value.
P values were determined by the chi-square test by comparison of the results for the Toxoplasma IgG-positive sera (group A; n = 50) and the IgG-negative sera (group C; n = 27).
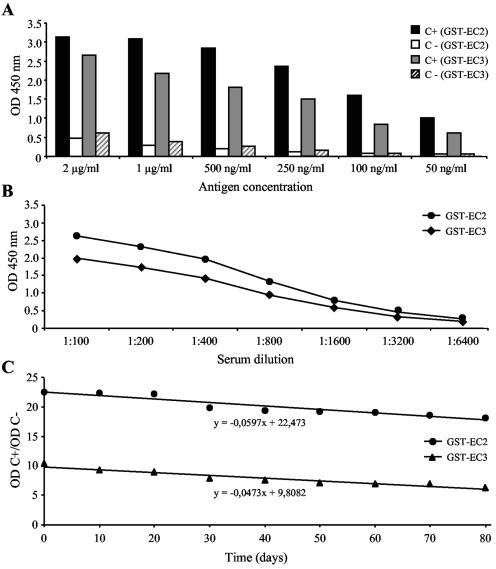
The purified proteins were chemically modified by biotinylation, resulting in 1.4 biotin molecules/molecule for GST-EC2 and 1.3 biotin molecules/molecule for GST-EC3. As shown in Fig. 3, the biotin-labeled antigens specifically reacted with Toxoplasma-specific IgM antibodies when a wide range of antigen concentrations (Fig. 3A) or serum dilutions (Fig. 3B) were used. The thermal stabilities of the chimeric antigens were analyzed. The time limits for the display of 50% of the Toxoplasma-specific IgM immunoreactivity were 189 and 97 days for the GST-EC2 and GST-EC3 antigens, respectively (Fig. 3C).
FIG. 3.
Immunoreactivities of the biotin-labeled chimeric antigens. (A) IgM Rec-ELISA performance with the biotin-labeled GST-EC2 and GST-EC3 antigens with Toxoplasma IgM-positive (C+) or IgM-negative (C−) serum samples (diluted 1:100) by using different antigen concentrations. (B) IgM Rec-ELISA performance with chimeric antigens (concentration, 500 ng/ml) assayed with different dilutions of a Toxoplasma IgM-positive serum. (C) Chimeric antigens (concentration, 250 ng/ml) were stored at 4°C intervals and assayed over time by the IgM Rec-ELISA. The results are expressed as the ratio of the OD measured with the Toxoplasma IgM-positive and -negative sera (OD C+ and OD C−, respectively).
Table 3 shows the IgM results with sera from adults. The proportions of IgM-reactive sera were 98% and 84% by use of the biotin-labeled GST-EC2 and GST-EC3 antigens, respectively. None of the Toxoplasma IgM-negative sera reacted with the chimeric products (data not shown). Table 4 shows the performance characteristics of commercial assays with lysed, whole-cell Toxoplasma antigen (ELFA-Toxo-IgM and ETI-TOXOK-M Reverse-PLUS) in comparison to the results obtained with the biotin-labeled GST-EC2 and GST-EC3 antigens (IgM Rec-ELISAs). Both specificity and positive predictive values reach the maximum (100%) when the biotin-labeled antigen immunoassays were used. Notably, the ETI-TOXOK-M test as well as the GST-EC2 IgM assay displayed identical diagnostic characteristics.
TABLE 3.
Immunoreactivities of chimeric antigens with IgM antibodies in sera from adults with acquired T. gondii infectiona
| Serum sample | IgG level (IU/ml) | Cutoffb
|
IgM Rec-ELISA cutoffc
|
||
|---|---|---|---|---|---|
| ELFA-Toxo-IgM | ETI-TOXOK-M | GST-EC2 | GST-EC3 | ||
| B1 | 28 | 5.37 | 2.74 | 2.950 | 1.948 |
| B2 | 255 | 3.86 | 1.49 | 0.546 | 0.498 |
| B3 | 78 | 3.01 | 1.38 | 0.471 | 0.867 |
| B4 | 1,358 | 2.28 | 1.17 | 0.464 | 0.453 |
| B5 | 178 | 2.31 | 1.27 | 0.598 | 0.406 |
| B6 | 155 | 2.00 | 0.97 | 0.993 | 0.720 |
| B7 | 109 | 3.20 | 1.76 | 0.794 | 0.642 |
| B8 | 99 | 3.16 | 1.78 | 0.572 | 0.389 |
| B9 | 103 | 2.28 | 1.34 | 1.056 | 1.222 |
| B10 | 85 | 2.22 | 1.44 | 0.930 | 0.704 |
| B11 | 70 | 1.01 | 0.79 | 0.416 | 0.376 |
| B12 | 26 | 1.34 | 0.93 | 0.392 | 0.461 |
| B13 | 36 | 1.22 | 0.86 | 0.532 | 0.499 |
| B14 | 156 | 0.99 | 0.58 | 0.534 | 0.833 |
| B15 | 204 | 0.93 | 0.90 | 0.810 | 0.710 |
| B16 | 133 | 1.13 | 0.85 | 0.465 | 0.322 |
| B17 | 183 | 1.14 | 0.82 | 0.325 | 0.327 |
| B18 | 242 | 0.90 | 0.71 | 0.497 | 0.500 |
| B19 | 80 | 1.00 | 0.79 | 0.444 | 0.706 |
| B20 | 258 | 1.40 | 0.88 | 2.678 | 0.484 |
| B21 | 278 | 1.69 | 1.06 | 0.703 | 0.509 |
| B22 | 246 | 1.25 | 0.76 | 1.094 | 0.780 |
| B23 | 59 | 1.23 | 0.71 | 0.495 | 1.499 |
| B24 | 38 | 0.78 | 0.87 | 0.584 | 0.455 |
| B25 | 130 | 0.76 | 0.92 | 0.562 | 0.545 |
| B26 | 262 | 0.84 | 0.65 | 0.649 | 0.551 |
| B27 | 168 | 0.96 | 0.85 | 1.439 | 0.938 |
| B28 | 126 | 0.78 | 0.80 | 2.475 | 1.160 |
| B29 | 197 | 1.38 | 0.61 | 0.544 | 0.358 |
| B30 | 127 | 0.86 | 0.52 | 0.847 | 0.531 |
| B31 | 72 | 1.28 | 0.93 | 1.756 | 0.891 |
| B32 | 130 | 0.77 | 0.71 | 0.505 | 0.381 |
| B33 | 439 | 1.00 | 0.66 | 0.834 | 0.464 |
| B34 | 83 | 0.66 | 1.32 | 1.162 | 0.989 |
| B35 | 178 | 0.89 | 0.87 | 0.694 | 0.487 |
| B36 | 560 | 0.86 | 0.69 | 0.817 | 0.628 |
| B37 | 223 | 0.96 | 0.73 | 0.531 | 0.819 |
| B38 | 242 | 0.98 | 0.41 | 0.379 | 0.318 |
| B39 | 118 | 1.16 | 0.84 | 0.420 | 0.380 |
| B40 | 232 | 1.39 | 1.01 | 0.490 | 0.467 |
| B41 | 213 | 1.03 | 1.05 | 0.750 | 0.822 |
| B42 | 243 | 1.06 | 0.97 | 0.534 | 0.502 |
| B43 | 154 | 0.75 | 0.73 | 0.455 | 0.337 |
| B44 | 35 | 1.90 | 1.51 | 0.383 | 1.008 |
| B45 | 667 | 0.85 | 1.01 | 0.366 | 0.285 |
| B46 | 275 | 0.95 | 0.99 | 0.411 | 0.544 |
| B47 | 157 | 1.93 | 1.36 | 0.382 | 0.464 |
| B48 | 1,037 | 1.08 | 0.51 | 0.385 | 0.301 |
| B49 | 92 | 1.31 | 0.68 | 0.537 | 0.427 |
| B50 | 255 | 0.69 | 0.69 | 0.354 | 0.801 |
Serum samples (samples B1 to B50) were analyzed by IgM Rec-ELISAs with individual GST-EC2 and GST-EC3 antigens or commercial assays with whole-cell Toxoplasma antigens (ELFA-IgM and ETI-TOXOK-M).
The cutoff values for the commercial ELFA-Toxo-IgM and ETI-TOXOK-M assays were 0.65 and 0.45, respectively, as indicated by the manufacturers. Boldface type, values greater than the cutoff value.
The cutoff values for the IgM Rec-ELISA with the GST-EC2 and GST-EC3 antigens, calculated with the IgM-negative sera (groups A and C; n = 40), were 0.34 and 0.38, respectively. Boldface type, values greater than the cutoff value.
TABLE 4.
Diagnostic performance of the IgM-capture assays with recombinant chimeric antigens or the whole-cell Toxoplasma antigena
| Diagnostic testb | Pc | Sensitivity (%) | Specificity (%) | Agreement (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| ELFA Toxo-IgM | <0.0001 | 100 | 100 | 100 | 100 | 100 |
| ETI-TOXOK-M | <0.0001 | 98.0 | 100 | 98.9 | 100 | 97.6 |
| EC2 IgM Rec-ELISA | <0.0001 | 98.0 | 100 | 98.9 | 100 | 97.6 |
| EC3 IgM Rec-ELISA | <0.0001 | 84.0 | 100 | 91.1 | 100 | 83.3 |
PPV, positive predictive value; NPV, negative predictive value.
The ELFA Toxo-IgM assay was used as the “reference test” to evaluate the performances of the other assays (ETI-TOXO and Rec-ELISAs).
P values were determined by the chi-square test by comparison of the results for the Toxoplasma IgM-positive sera (group B; n = 50) and the IgM-negative sera (groups A and C; n = 40).
The immunoreactivities of the biotin-labeled GST-EC2 and GST-EC3 antigens with IgM antibodies in sera from infants born to mothers with primary T. gondii infection during pregnancy were assayed. Table 5 shows the characteristics of the infected patient cohort. The specific levels of anti-Toxoplasma IgG, as determined by ELFA-IgG (26), ranged from 28 to 1,147 IU/ml for sera from infected infants and from 19 to 170 IU/ml for sera from uninfected subjects. The results of the IgM Rec-ELISAs with individual sera from infected infants are summarized in Table 5. Overall, the proportions of IgM-reactive sera were 70% (14/20) and 50% (10/20) by use of the GST-EC2 and GST-EC3 antigens, respectively. In contrast, only 7 of 20 infected infants (35%) had positive results when the ELFA-IgM and ETI-TOXOK-M assays were used. None of the sera from the uninfected infants recognized the GST-EC2 and GST-EC3 antigens in the IgM Rec-ELISA or were positive by the commercial assays (data not shown).
TABLE 5.
Toxoplasma-specific IgM reactivities of serum samples from infants with congenital toxoplasmosisa
| Patient no. | Time after birth (wk) | Onsetb | IgG level (IU/ml) | Cutoffc
|
IgM Rec-ELISA cutoffd
|
||
|---|---|---|---|---|---|---|---|
| ELFA-Toxo-IgM | ETI-TOXOK-M | GST-EC2 | GST-EC3 | ||||
| T1 | 1 | B | 169 | 6.41 | 2.66 | 2.479 | 0.542 |
| T2 | 2 | B | 988 | 0.73 | 0.15 | 0.360 | 0.270 |
| T3 | 2 | Sub | 300 | 0.09 | 0.13 | 0.212 | 0.209 |
| T4 | 3 | Sub | 57 | 0.05 | 0.23 | 0.206 | 0.211 |
| T5 | 3 | Sub | 124 | 0.13 | 1.62 | 0.641 | 1.103 |
| T6 | 4 | Sub | 218 | 0.04 | 0.09 | 0.452 | 0.269 |
| T7 | 4 | S | 157 | 2.61 | 1.62 | 1.522 | 0.225 |
| T8 | 4 | S | 172 | 3.98 | 1.50 | 1.804 | 0.353 |
| T9 | 5 | S | 1147 | 0.07 | 0.10 | 0.519 | 0.206 |
| T10 | 5 | B | 47 | 0.11 | 0.12 | 0.272 | 0.276 |
| T11 | 6 | Sub | 28 | 0.10 | 0.18 | 2.617 | 0.731 |
| T12 | 6 | Sub | 136 | 0.07 | 0.11 | 0.314 | 0.216 |
| T13 | 7 | S | 209 | 0.88 | 0.47 | 0.683 | 0.217 |
| T14 | 8 | Sub | 43 | 0.06 | 0.07 | 0.196 | 0.213 |
| T15 | 8 | B | 160 | 0.82 | 0.08 | 0.206 | 0.219 |
| T16 | 8 | B | 64 | 0.02 | 0.40 | 0.231 | 0.228 |
| T17 | 8 | Sub | 145 | 0.31 | 0.57 | 0.985 | 0.314 |
| T18 | 9 | Sub | 300 | 6.37 | 1.30 | 0.548 | 0.315 |
| T19 | 12 | Sub | 196 | 0.05 | 0.17 | 0.463 | 0.268 |
| T20 | 12 | Sub | 75 | 0.05 | 0.07 | 0.237 | 0.222 |
Serum samples (samples T1 to T20) were analyzed by IgM Rec-ELISAs with GST-EC2 and GST-EC3 antigens or by commercial assays (ELFA-IgM and ETI-TOXO-M).
Severity of clinical onset: S, severe; B, benign; Sub, subclinical.
The cutoff values for the ELFA-IgM and ETI-TOXO-M assays were 0.65 and 0.41, respectively, as indicated by manufacturers. Boldface type, values greater than the cutoff value.
The cutoff values for the IgM Rec-ELISA with the GST-EC2 and GST-EC3 antigens, calculated with sera from uninfected infants (n = 10), were 0.25 and 0.26, respectively. Boldface type, values greater than the cutoff value.
DISCUSSION
In this study we evaluated the diagnostic utility of IgG and IgM Rec-ELISAs with different antigenic regions of T. gondii gene products, which were combined into chimeric antigens by genetic engineering in order to replace the tachyzoite antigen in serological tests. The main objective of our work was, therefore, the provision of “artificial” antigens and their use for the development of selective diagnostic immunoassays.
Six distinct antigenic regions of T. gondii, the MIC2, MIC3, SAG1, GRA3, GRA7, and M2AP proteins (3, 5), were used to construct the GST-EC2 and GST-EC3 antigens. By using a standard bacterial expression system, the chimeric antigens were purified under native conditions in large amounts and were found to have good solubilities and elevated purification yields, which are fundamental prerequisites for the commercial use of a recombinant protein. Each protein fragment of the individual T. gondii polypeptides retained its antigenic property when it was assembled into the GST-EC2 and GST-EC3 antigens, indicating that the molecular fusion does not interfere with the binding of each antigen fragment composing the chimera to the corresponding specific antibody. Moreover, it was possible to label the chimeric proteins by biotinylation without affecting the interaction between the antigens and the antibodies, emphasizing the usefulness of such recombinant proteins for diagnostic applications. Finally, our results demonstrated that the GST-EC2 and GST-EC3 antigens have excellent thermal stability, a characteristic that is often necessary when the antigen is used in a soluble form in automated systems (e.g., the reverse IgM assay).
The immunoreactivities of the chimeric antigens were assessed with IgG and IgM antibodies present in sera from adults with acquired T. gondii infection. IgG antibodies in 100% of the serum samples from the Toxoplasma IgG-positive subjects, as measured by the standard whole-cell tachyzoite assay ELFA-IgG, specifically reacted with the recombinant GST-EC2 and GST-EC3 antigens. By contrast, none of the IgG Rec-ELISAs with single antigenic domains detected all of the Toxoplasma IgG-positive samples, suggesting that multiple epitopes from different antigens need to be present to recognize antibodies present in a particular disease state. Also, the larger sizes of the chimeric proteins compared with the sizes of the single recombinant antigens might result in better adherence to the microtiter plate, thus resulting in improved assay sensitivity.
The results of the IgM Rec-ELISAs were compared with those obtained with commercial assays with the whole-cell tachyzoite antigen. Overall, 98% of the serum samples (49/50) with specific anti-Toxoplasma IgM antibodies, as measured by the standard whole-cell tachyzoite assay ELFA-IgM, were found to be positive by the IgM Rec-ELISA; and the same result was obtained with the commercial IgM-capture assay ETI-TOXOK-M. Only one false-negative result was observed by use of the GST-EC2 antigen in the IgM Rec-ELISA (Table 3, serum sample B17). However, the false-negative result could be avoided by slightly lowering the cutoff value of the assay, although this resulted in one false-positive result among the IgM-negative subjects (data not shown). Therefore, a higher cutoff value was used, despite the reduced assay sensitivity.
Several previous studies have demonstrated the usefulness of recombinant antigens for the serological diagnosis of T. gondii infection (1, 4, 8, 17, 22, 27, 31). However, the exact composition of a recombinant protein cocktail representative of the antigenic repertoire present in the tachyzoite soluble extract and usable for the detection of both IgG and IgM antibodies in an immunoassay remains an open question. Our results demonstrated that the IgG and IgM Rec-ELISAs with chimeric antigens have performance characteristics comparable to those of assays with the whole-cell tachyzoite antigen. Consequently, the major advantage of using a chimeric antigen for antibody detection rather than the existing commercial assays would be a more “standardized” antigen.
The chimeric antigen immunoreactivity was assessed with IgM antibodies in sera from infants with congenital T. gondii infection. Overall, 70% of the infants (14/20) with congenital toxoplasmosis were found to be positive by IgM Rec-ELISA analysis (Table 5, results for GST-EC2), whereas the ELFA-IgM or the ETI-TOXOK-M whole-cell immunoassays found only 35% (7/20) of the infected infants to be positive. These results thus demonstrate that assays based on chimeric antigens improve the diagnosis of newborns with congenital toxoplasmosis.
In conclusion, this study demonstrates that the use of recombinant chimeric antigens is effective in distinguishing T. gondii-infected individuals from uninfected individuals and that assays with such antigens have performance characteristics comparable to or even better than those of assays that use the whole-cell tachyzoite antigen. Such antigens could provide the basis for standardized commercial immunoassays for the serodiagnosis of toxoplasmosis.
Acknowledgments
We are grateful to Fabrizio Bonelli for providing us with the anti-human IgM monoclonal antibody and for very helpful suggestions during this work. We acknowledge Eskild Petersen and Paola Del Porto for helpful discussions and Annalisa De Vita for secretarial assistance.
REFERENCES
- 1.Aubert, D., G. T. Maine, I. Villena, J. C. Hunt, L. Howard, M. Sheu, S. Brojanac, L. E. Chovan, S. F. Nowlan, and J. M. Pinon. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 38:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman, M. H., R. E. McCabe, S. Y. Wong, and J. S. Remington. 1995. Toxoplasma gondii, p. 2455-2475. In G. L. Mandel, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, 4th ed. Churchill Livingstone, Inc., New York, N.Y.
- 3.Beghetto, E., A. Spadoni, W. Buffolano, M. Del Pezzo, O. Minenkova, E. Pavoni, A. Pucci, R. Cortese, F. Felici, and N. Gargano. 2003. Molecular dissection of the human B-cell response against Toxoplasma gondii infection by lambda display of cDNA libraries. Int. J. Parasitol. 33:163-173. [DOI] [PubMed] [Google Scholar]
- 4.Beghetto, E., W. Buffolano, A. Spadoni, M. Del Pezzo, M. Di Cristina, O. Minenkova, E. Petersen, F. Felici, and N. Gargano. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41:5414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beghetto, E., H. V. Nielsen, P. Del Porto, W. Buffolano, S. Guglietta, F. Felici, E. Petersen, and N. Gargano. 2005. A combination of antigenic regions of Toxoplasma gondii microneme proteins induce protective immunity against oral infection with parasite cysts. J. Infect. Dis. 191:637-645. [DOI] [PubMed] [Google Scholar]
- 6.Bermudes, D., J. F. Dubremetz, A. Achbarou, and K. A. Joiner. 1994. Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol. Biochem. Parasitol. 68:247-257. [DOI] [PubMed] [Google Scholar]
- 7.Buffolano, W., M. Lappalainen, L. Hedman, F. Ciccimarra, M. Del Pezzo, R. Rescaldani, N. Gargano, and K. Hedman. 2004. Delayed maturation of IgG avidity in congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 23:825-830. [DOI] [PubMed] [Google Scholar]
- 8.Buffolano, W., E. Beghetto, M. Del Pezzo, A. Spadoni, M. Di Cristina, E. Petersen, and N. Gargano. 2005. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 43:5916-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141:3584-3591. [PubMed] [Google Scholar]
- 10.Candolfi, E., M. H. Bessieres, P. Mart, B. Cimon, F. Gandilhon, H. Pelloux, and P. Thulliez. 1993. Determination of a new cut-off value for the diagnosis of congenital toxoplasmosis by detection of specific IgM in an enzyme immunoassay. Eur. J. Clin. Microbiol. Infect. Dis. 12:396-398. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, D., M. Wallon, F. Peyron, E. Petersen, C. S. Peckham, and R. Gilbert. 1999. Mother to child transmission of toxoplasmosis: risk estimates for clinical counseling. Lancet 353:1829-1833. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, H. G., S. Stachelhaus, M. Sahm, H. M. Meyer, and G. Reichmann. 1998. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91:251-262. [DOI] [PubMed] [Google Scholar]
- 13.Fricker-Hidalgo, H., H. Pelloux, C. Racinet, M. Bost, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1996. Congenital toxoplasmosis: specific IgM in fetal blood, cord blood and in the newborn. Ann. Biol. Clin. 54:165-168. [PubMed] [Google Scholar]
- 14.Garcia-Règuet, N., M. Lebrun, M. N. Fourmaux, O. Mercereau-Puijalon, T. Mann, J. M. Beckers, B. Samin, J. Van Beeumen, D. Bout, and J. F. Dubremetz. 2000. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface on the host cells and the surface of the parasite. Cell. Microbiol. 2:353-364. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, R., D. Dunn, M. Wallon, M. Hayde, A. Prusa, M. Lebech, T. Kortbeek, F. Peyron, A. Pollak, and E. Petersen. 2001. Ecological comparison of the risks of mother-to-child transmission and clinical manifestations of congenital toxoplasmosis according to prenatal treatment protocol. Epidemiol. Infect. 127:113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, R., L. Grass, and the European Multicentre Study on Congenital Toxoplasmosis. 2003. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. Br. J. Obstet. Gynaecol. 110:112-120. [DOI] [PubMed] [Google Scholar]
- 17.Harning, D., J. Spenter, A. Metsis, J. Vuust, and E. Petersen. 1996. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofgartner, W. T., S. R. Swanzy, R. M. Bacina, J. Condon, M. Gupta, P. E. Matlock, D. L. Bergeron, J. J. Plorde, and F. R. Fritsche. 1997. Detection of immunoglobulin G (IgG) and IgM antibodies to Toxoplasma gondii: evaluation of four commercial immunoassay systems. J. Clin. Microbiol. 35:3313-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohlfeld, P., F. Daffos, P. H. Thulliez, C. Aufrant, J. Couvreur, J. MacAleese, D. Descombey, and F. Forestier. 1989. Fetal toxoplasmosis: outcome of pregnancy and infant follow-up after in utero treatment. J. Pediatr. 115:765-769. [DOI] [PubMed] [Google Scholar]
- 20.Hohlfeld, P., F. Daffos, J. M. Costa, P. H. Thuillez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, D., M. Vercammen, and E. Saman. 1999. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin. Diagn. Lab. Immunol. 6:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, S., G. Galvan, F. G. Araujo, Y. Suzuki, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7:781-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liesenfeld, O., C. Press, R. Flanders, R. Ramirez, and J. S. Remington. 1996. Study of Abbott Toxo IMx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J. Clin. Microbiol. 34:2526-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minenkova, O., A. Pucci, E. Pavoni, A. De Tomassi, P. Fortugno, N. Gargano, M. Cianfriglia, S. Barca, S. De Placido, A. Martignetti, F. Felici, R. Cortese, and P. Monaci. 2003. Identification of tumor-associated antigens by screening phage-displayed human cDNA libraries with sera from tumor patients. Int. J. Cancer 106:534-544. [DOI] [PubMed] [Google Scholar]
- 26.Pelloux, H., P. Ciapa, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1993. Evaluation du systeme VIDAS pour le diagnostic serologique de la toxoplasmose. Ann. Biol. Clin. 50:875-878. [PubMed] [Google Scholar]
- 27.Pietkiewicz, H., E. Hiszyska-Sawicka, J. Kur, E. Petersen, H. V. Nielsen, M. Stankiewicz, I. Andrzejewska, and P. Myjak. 2004. Usefulness of Toxoplasma gondii recombinant antigens in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 42:1779-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabenau, K. E., A. Sohrabi, A. Tripathy, C. Reitter, J. W. Ajioka, T. M. Tomley, and V. B. Carruthers. 2001. TgM2AP participates in Toxoplasma gondii invasion of host cells and is tightly associated with the adhesive protein TgMIC2. Mol. Microbiol. 41:537-547. [DOI] [PubMed] [Google Scholar]
- 29.Remington, J. S., R. McLeod, P. Thuillez, and G. Desmonts. 2001. Toxoplasmosis, p. 205-346. In J.S. Remington and J. Klein (ed.), Infectious diseases of the foetus and newborn infant, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 30.Remington, J. S., P. Thuillez, and J. G. Montoya. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, Y., R. Ramirez, C. Press, S. Li, S. Parmley, P. Thulliez, and J. S. Remington. 2000. Detection of immunoglobulin M antibodies to P35 antigen of Toxoplasma gondii for serodiagnosis of recently acquired infection in pregnant women. J. Clin. Microbiol. 38:3967-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallon, M., L. Kodjikian, C. Binquet, J. Garweg, J. Fleury, C. Quantin, and F. Peyron. 2004. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 113:1567-1572. [DOI] [PubMed] [Google Scholar]
- 33.Wan, K. L., V. B. Carruthers, L. D. Sibley, and J. W. Ajioka. 1997. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol. 84:203-214. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, M., J. S. Remington, C. Clavet, G. Varney, C. Press, D. Ware, et al. 1997. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J. Clin. Microbiol. 35:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]