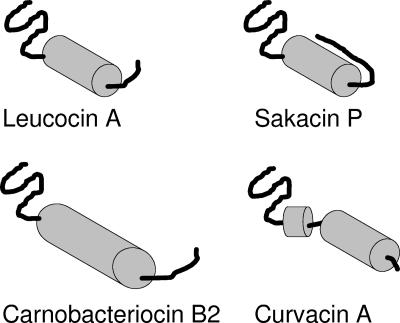
FIG. 2.
Schematic presentation of the four class IIa bacteriocins for which 3D structures have been determined by NMR, i.e., leucocin A (67), carnobacteriocin B2 (189), sakacin P (176), and curvacin A (79). Due to sequence similarities among these class IIa bacteriocins, it is assumed that the 3D structures of their N-terminal beta-sheet-like structures are relatively similar despite the fact that their NMR structures display some variation in this region. It is also assumed that the C-terminal tails following the central alpha helices of leucocin A and carnobacteriocin B2 most likely form a hairpin-like structure together with the alpha helix, despite the fact that this could not be judged from the NMR structural analyses. This hairpin-like structure has been seen only for the structurally stabilized sakacin P variant (176).