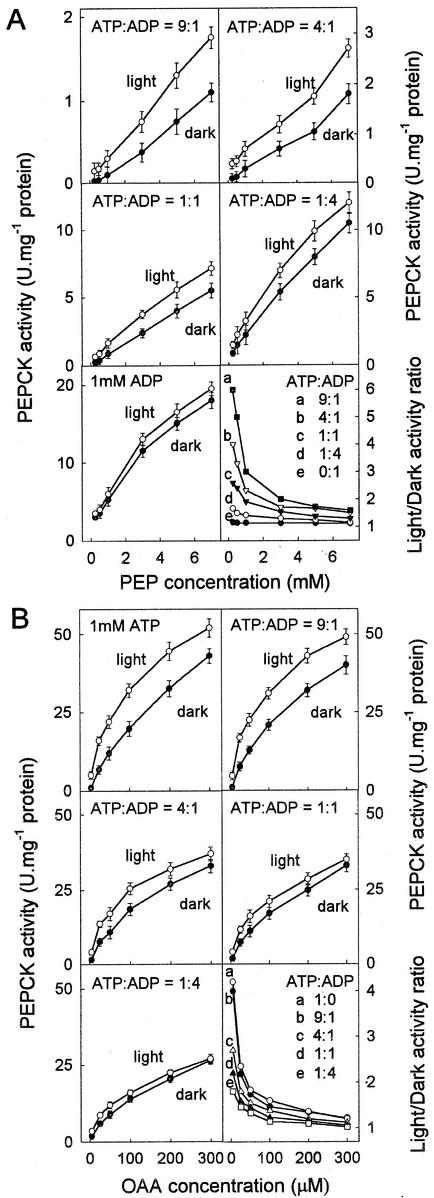
Figure 3.
Effects of phosphorylation on the activity of PEPCK. A, The effect of phosphorylation on the carboxylase activity of PEPCK. The affinity of the phosphorylated and non-phosphorylated forms of the enzyme for PEP was determined at different ratios of ATP:ADP. B, The effect of phosphorylation on the decarboxylase activity of PEPCK. The affinity of the phosphorylated and non-phosphorylated forms of the enzyme for OAA was determined with different ratios of ATP to ADP. In both A and B the total concentration of adenylates was 1 mm. The rate at zero substrate concentration was always zero. To illustrate the interaction between phosphorylation state, ATP:ADP ratio and concentration of OAA or PEP the ratio of the activity of the non-phosphorylated (light) to phosphorylated (dark) enzyme for each value of ATP:ADP was plotted against the OAA or PEP concentration.