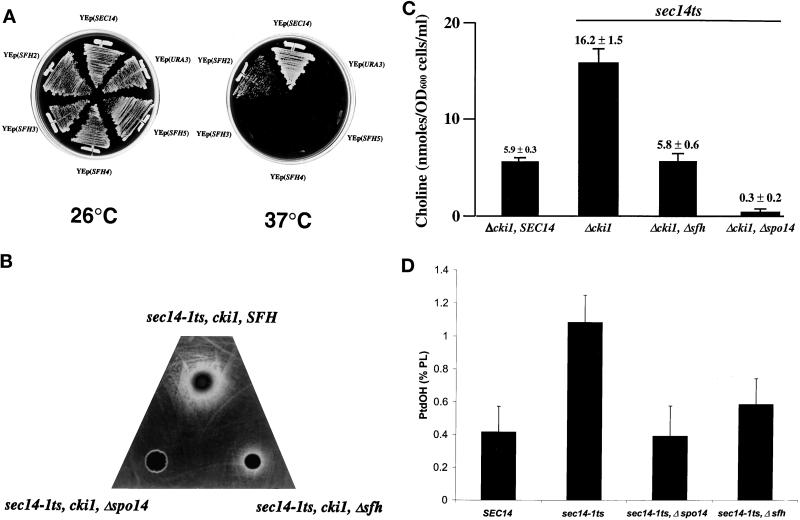
Figure 5.
SFH gene products contribute to PLD activity in vivo. (A) PLD requirement for YEp(SFH)-mediated rescue of sec14-associated growth defects. The sec14-1ts, Δspo14 strain CTY1079 was transformed with the indicated YEp(SFH) plasmids and YEp(SEC14) and YEp(URA3) plasmids as positive and negative controls, respectively. The corresponding transformants were streaked for isolated colonies on YPD agar and incubated at temperatures permissive (26°C) or restrictive (37°C) for the sec14-1ts, Δspo14 strain, as indicated. Results were scored after 48 h of incubation. YEp(SFH2) elicited a clear phenotypic rescue of sec14-1ts growth defects, whereas YEp(SFH3), YEp(SFH4), and YEp(SFH5) were unable to effect any relief of the sec14-associated growth defects in the absence of functional PLD activity. (B) PLD activation in Δsfh yeast strains as measured by choline excretion. Equal cell numbers of the sec14-1ts, cki1 strain CTY160 and its congenic sec14-1ts, cki1, Δsfh derivative CTY1427 were spotted on a lawn of the choline auxotrophic strain CTY1289 plated onto choline-free minimal agar. The plate was incubated at 30°C for 48 h. An inoculum of the isogenic Δspo14 derivative strain CTY1099 was also spotted onto the indicator lawn, and the absence of choline cross-feeding of the indicator establishes that the choline excreted by the CTY160 and CTY1289 cells was generated by the action of PLD. (C) Chemical quantification of choline excretion. The appropriate yeast strains were grown to midlogarithmic growth phase in minimal defined medium supplemented with inositol (100 μM) but lacking choline at 26°C. The cells were harvested, washed, and resuspended in fresh medium and incubated at 33.5°C for 3 h. A choline oxidase–coupled chemical assay was used to measure the choline concentration of culture supernatant (see MATERIALS AND METHODS). Relevant genotypes of the strains are given below the bars. Data represent triplicate determinations from at least three independent experiments. (D) PLD activation in Δsfh yeast strains as measured by PtdOH production. The appropriate yeast strains were radiolabeled to steady state with [32P]orthophosphate (10 μCi/ml) at 26°C in inositol- and choline-replete minimal medium (Xie et al., 1998). Cells were subsequently washed and incubated in radiolabel-free medium for 3 h at 33.5°C, and phospholipids were extracted and resolved (Xie et al., 1998). PtdOH was identified and quantified by phosphorimaging. Values are expressed as [32P (cpm) incorporated into PtdOH divided by total 32P (cpm) incorporated into extractable phospholipid] times 100%. The data were normalized by measuring relative rates of phosphate incorporation into phospholipid as a function of cell number for each strain. Under these experimental conditions, the following values of 32P (cpm) incorporation into extractable phospholipid per OD600 cells were obtained: CTY182 (SEC14, SFH), 8500 − 245; CTY1-1A (sec14-1ts, SFH), 17,000 − 1470; CTY1079 (sec14-1ts, Δspo14), 24,000 − 2400; CTY1118 (sec14-1ts, Δsfh), 15,000 − 645. These data represent the averages of at least three independent experiments.