Abstract
OBJECTIVES
Both clinical trials and observational studies of persons with HIV infection commonly include health-related quality of life (HRQOL) measures, but less is known about the relation of HRQOL to survival among persons with HIV since the development of effective antiretroviral treatment.
DESIGN/PARTICIPANTS
Prospective cohort study of a national probability sample of 2,864 adults receiving HIV care.
INDEPENDENT VARIABLES
The main independent variables were derived from the HIV Cost and Services Utilization Study (HCSUS) HRQOL measure, and include physical and mental HRQOL summary scores (divided into quartiles) constructed from the following items administered at baseline: physical functioning (9 items, α=0.91), role functioning (2 items, α=0.85), pain (2 items, α=0.84), general health perceptions (3 items, α=0.80), emotional well-being (7 items, α=0.90), social functioning (2 items, α=0.82), energy (2 items, α=0.74), and a single-item measure of disability days (days in bed for at least 0.5 day due to health).
MAIN OUTCOME VARIABLE
Death between January 1996 and December 1999.
ANALYSIS
Descriptive and multivariate adjusted Cox proportional hazards regression analysis of survival by physical and mental HRQOL.
RESULTS
By December 1999, 17% of the sample had died. In unadjusted analysis, persons in the higher quartiles of physical HRQOL, as well as those in the higher quartiles of mental HRQOL at baseline, had significantly better survival than those in lower quartiles. In multiple regressions controlling for a number of sociodemographic and clinical variables, the hazard of death for those in the highest quartile of physical HRQOL was one quarter of that for those in the lowest quartile. This difference was similar in magnitude to that observed for being on highly active antiretroviral therapy versus no antiretrovirals in this population. By contrast, there was no unique association of mental HRQOL with survival.
CONCLUSIONS
Physical HRQOL added prognostic information over and above the sociodemographic and routinely available clinical data such as CD4 count and stage of HIV infection. Measurement of HRQOL, which is often performed to identify problems or assess outcomes, is also useful prognostically.
Keywords: HIV, AIDS, quality of life, survival, outcomes
Recent advances in HIV treatment have provided great hope for longer survival and better health-related quality of life (HRQOL) for the nearly 1 million persons in the United States infected with HIV.1,2 However, little is known about the relation of HRQOL to survival among persons with HIV across the United States. Health-related quality of life (HRQOL) consists of the ability to perform daily activities, such as walking and working, and well-being, or lack thereof, because of health. It covers broad aspects of physical and mental health that are rarely assessed directly in routine medical encounters.3 HIV infection is an important chronic condition in which to study HRQOL and survival, because of the myriad mental and physical manifestations over the course of the illness, and because of the high mortality rate. Both clinical trials and observational studies of persons with HIV infection often include HRQOL measures to evaluate the simultaneous effects of clinical interventions, treatment side effects, and disease impact over time.4–7 Some studies have also shown a relation of HRQOL with laboratory and other clinical end points.8–10
While it is generally acknowledged that it is important for providers to be aware of these multiple domains of health in HIV, few studies have examined the extent to which HRQOL measures predict the outcome of HIV infection. In particular, it would be useful to know whether HRQOL predicted survival, particularly if it added information about survival that was distinct from that provided by traditional clinical variables such as CD4 count, AIDS-defining illnesses, and use of highly active antiretroviral therapy (HAART). Most of the prior literature linking some dimensions of HRQOL with survival predates the HAART era and therefore may not apply to current experience. Since the advent of HAART, outcomes of HIV infection have been improved, and the length of life for persons with HIV has increased.11 In the context of HIV as a chronic disease, the clinical usefulness of HRQOL measures as predictors of survival has not been examined extensively.
To examine whether generic HRQOL measures can predict survival among persons with HIV infection in the United States, we analyzed data from the HIV Cost and Services Utilization Study (HCSUS). The HCSUS is a nationally representative cohort of persons with HIV infection who were receiving care at least every 6 months in the contiguous United States starting in 1996. The purpose of this study was to examine whether physical and mental HRQOL measures were associated with mortality at follow-up after controlling for markers of disease stage and treatment and sociodemographic variables. If HRQOL is associated with mortality in HIV, then measuring HRQOL may be useful as a risk stratification tool in clinical practice.
METHODS
Study Sample
Full details of the HCSUS sampling design are presented elsewhere.12 In brief, the reference population was persons at least 18 years old with known HIV infection who made at least one visit, in the context of regular or ongoing care, to a nonmilitary, nonprison medical provider (other than an emergency department) in the contiguous United States, during the period January 5 to February 29, 1996. The HCSUS used a three-stage sampling design, in which geographical areas, medical providers, and patients were sampled. In the first stage, we sampled 28 metropolitan areas and 25 clusters of rural counties within the United States. In the second stage, we sampled a total of 148 urban and 51 rural providers. In the third stage, we sampled patients from de-identified lists of all eligible patients who visited participating providers during January and February 1996. We constructed several weights to adjust for the differential selection probabilities across subgroups of the population, one to adjust for nonresponse, and another to adjust for the fact that some patients had more than one opportunity to enter the sample. Applying the weights permits inference to the population represented by the baseline sample. We sampled 4,042 eligible subjects, of whom 2,864 (71%) completed full baseline interviews. After obtaining informed consent, all interviews were conducted using computer-assisted personal interviewing (CAPI) programs designed for this study. Baseline interviews were conducted between January 1996 and April 1997. Patients were followed through December 1999 to ascertain survival. The median duration of follow-up was 41 months.
Mortality Assessment
We used several methods to assess death. Field staff interviewed participants' family, friends, or other contacts and examined newspaper obituaries. In addition, mortality searches were done under Equi-Fax Government and Special Systems (now known as Choice Point), a national death registry. Equi-Fax identifies mortality status through the use of a national database that compiles information from death certificates processed in the United States. Lastly, we conducted mortality searches through the National Death Index. Mortality status for patients was checked in these databases by matching the patients' social security numbers with those in the national database. As of December 31, 1999, 486 (17%) of the 2,864 patients enrolled at baseline were deceased. There were 19 (< 4% of deceased,<1% of total) patients for whom the date of death was only known within a range (5.5–15.1 months); we imputed the date of death randomly selecting a date in the interval during which death was known to occur. The results of the analysis were not sensitive to whether missing values were imputed at the high versus low end of the interval.
Measures of HRQOL
The HCSUS HRQOL survey included multi-item measures of physical functioning (9 items, α=0.91), role functioning (2 items, α=0.85), bodily pain (2 items, α=0.84), general health perceptions (3 items, α=0.80), emotional well-being (7 items,α=0.90), social functioning (2 items, α=0.82), and energy (2 items, α=0.74) at baseline. We also included a single-item measure of disability days (days in bed for at least 0.5 day due to health). As previously reported, we derived physical and mental HRQOL summary indices from the physical functioning, role functioning, pain, general health perceptions, emotional well-being, social functioning, energy, and disability days measures using factor analysis of these 8 scores.2 The summary indices were transformed linearly to T-scores (mean, 50; standard deviation [SD], 10; higher scores represent better health)13 and divided into quartiles for further analysis. In addition, we conducted analyses to assess the associations of the 8 original measures with survival.
Control Variables
In multivariate analyses, we adjusted for potential confounders, including age (18–34, 35–49, 50 and older), gender, race/ethnicity (non-Hispanic white, non-Hispanic black/African-American, Hispanic, other), educational attainment (less than high school degree, high school degree, some college, college degree or more), income ($0–$5,000, $5,001–$10,000, $10,001–$25,000, greater than $25,000), U.S. geographic region (northeast, south, midwest, and west), insurance status (private/fee-for-service, private/HMO, Medicare, Medicaid, uninsured), and HIV exposure group (intravenous drug use, male-to-male sexual contact, heterosexual contact, other). Lastly, we assessed the following control variables: CD4 count (>=500, 200–499, 50–199, 0–49), stage of HIV infection (Center for Disease Control and Prevention [CDC] Stages A, B, and C: asymptomatic, symptomatic, AIDS), and use of highly active antiretroviral medications (none, 1–2 non-HAART medications, HAART combinations). Our definition of HAART was based on the Department of Health and Human Services/Henry J. Kaiser Foundation Guidelines for the Use of Antiretroviral Agents in HIV-infected Adults and Adolescents as taking combinations of nucleoside reverse transcriptase inhibitors (NRTI; e.g., zidovudine and lamuvidine) plus certain protease inhibitors (PI; e.g., nelfinavir), combinations of PIs (e.g., ritonavir and saquinavir), or the combination of a PI plus a non–nucleoside reverse transcriptase inhibitor (NNRTI; e.g., delavirdine).14,15 All control variables were measured at baseline.
Analysis
Initially, we examined Kaplan-Meier tables and survival curves by quartile of physical and mental HRQOL scores. Differences in survival by quartile of HRQOL were tested statistically using the log rank test. Then we used Cox proportional hazards regression models to evaluate the association of physical and mental HRQOL with mortality after controlling for sociodemographic, clinical (CD4 count, CDC stage of HIV infection), and antiretroviral medication variables. Adjusted relative hazard ratios and 95% confidence intervals were computed to express the strength of association of being in each of the higher three HRQOL quartiles, compared to the lowest (fourth) quartile, with time to death. We also conducted a sensitivity analysis rescaling the continuous HRQOL measures so that 1 point=1 standard deviation. All analyses adjust for the complex multistage sampling design. Linearization methods in the survey analysis procedures of Stata 8.0 (Stata Corporation, College, Station, TX) were used in all models to account for clustering, stratification, and sampling weights, and to estimate hazard ratios, standard errors, and levels of significance.16
RESULTS
Baseline HRQOL and Subsequent Survival: Bivariate and Life Table Analysis
Of the 2,864 persons (representing a population of 231,400 nationally) with HIV who were interviewed at baseline, 17% had died by December 1999. Characteristics of the overall population have been previously reported.17 At baseline, the mean physical HRQOL summary score was 51.3 among those alive at follow-up, versus 43.5 among those who had died (Table 1), a highly significant difference. Similarly, the baseline mental HRQOL summary score was significantly higher among those who went on to survive the follow-up period versus those who did not. In addition, those who died during the follow-up period were more likely to be older, and have less education, low income, public insurance, other mode of HIV infection, CD4 counts less than 50, and an advanced stage of HIV infection, compared to those still alive at follow-up.
Table 1.
Baseline Characteristics of Those Alive Versus Those Dead at Follow-up in HCSUS
| n | Alive | Dead | P Value | |
|---|---|---|---|---|
| Physical HRQOL (mean, 95% CI) | 2,864 | 51.3 (50.4 to 52.3) | 43.5 (42.1 to 44.8) | .0001 |
| Mental HRQOL (mean, 95% CI) | 2,864 | 50.5 (49.8 to 51.3) | 47.4 (46.1 to 48.7) | .0001 |
| Age, % | .004 | |||
| 18–34 | 987 | 85.4 | 14.6 | |
| 35–49 | 1,591 | 82.9 | 17.1 | |
| 50≤ | 286 | 76.9 | 23.1 | |
| Gender, % | .46 | |||
| Male | 2,017 | 82.8 | 17.2 | |
| Female | 847 | 84.1 | 15.9 | |
| Race/ethnicity, % | .48 | |||
| White | 1,399 | 83.3 | 16.7 | |
| Black | 959 | 81.8 | 18.2 | |
| Hispanic | 415 | 84.1 | 15.9 | |
| Other | 91 | 88.8 | 11.2 | |
| Education, % | .003 | |||
| College degree | 526 | 85.9 | 14.1 | |
| Some college | 810 | 83.8 | 16.2 | |
| High school degree | 805 | 84.4 | 15.6 | |
| No high school degree | 723 | 78.7 | 21.3 | |
| Annual household income, % | .03 | |||
| >$25,000 | 779 | 86.4 | 13.6 | |
| $10,001–$25,000 | 736 | 83.3 | 16.7 | |
| $5,001–$10,000 | 740 | 78.7 | 21.3 | |
| $0–$5,000 | 609 | 83.7 | 16.3 | |
| Insurance status, % | .001 | |||
| Private/fee-for-service | 391 | 86.9 | 13.1 | |
| Private HMO | 474 | 87.4 | 12.6 | |
| Medicaid | 858 | 80.6 | 19.4 | |
| Medicare | 544 | 72.0 | 28.0 | |
| No insurance | 597 | 90.9 | 9.1 | |
| HIV exposure, % | .002 | |||
| Male-to-male sexual contact | 1,303 | 85.0 | 15.0 | |
| Injecting drug use | 696 | 79.1 | 20.9 | |
| Heterosexual contact | 578 | 87.7 | 12.3 | |
| Other mode | 287 | 73.8 | 26.2 | |
| Geographic region, % | .36 | |||
| West | 909 | 85.8 | 14.2 | |
| Northeast | 707 | 83.2 | 16.8 | |
| Midwest | 332 | 77.9 | 22.1 | |
| South | 916 | 82.5 | 17.5 | |
| CD4 count, % | .001 | |||
| > 500 | 253 | 94.3 | 5.7 | |
| 200–499 | 1,096 | 93.7 | 6.3 | |
| 50–199 | 854 | 84.2 | 15.8 | |
| <50 | 661 | 60.4 | 39.6 | |
| CDC stage of HIV infection, % | .001 | |||
| Asymptomatic | 243 | 94.0 | 6.0 | |
| Symptomatic | 1,495 | 90.6 | 9.4 | |
| AIDS | 1,126 | 70.2 | 29.8 | |
| Antiretroviral medications, % | .11 | |||
| No antiretrovirals | 530 | 86.1 | 13.9 | |
| 1 antiretroviral | 622 | 78.9 | 21.1 | |
| 2 or more antiretrovirals | 992 | 84.6 | 15.4 | |
| HAART | 720 | 82.6 | 17.4 |
Differences in the proportions of each baseline characteristic alive versus dead at follow-up were tested with χ2 tests. HCSUS, HIV Cost and Services Utilization Study; HRQOL, health-related quality of life; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval.
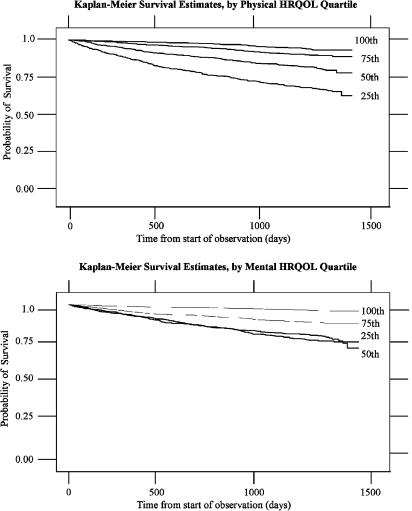
In Kaplan-Meier analysis, persons in the higher quartiles of physical health had significantly and monotonically better survival than those in lower quartiles (Fig. 1). The unadjusted 48-month survival probabilities for those in the first through fourth quartiles of physical HRQOL were 93%, 89%, 78%, and 63%, respectively. Similarly, those in higher quartiles of mental HRQOL had significantly better survival than those in lower quartiles, but the effect was not quite monotonic. The unadjusted 48-month survival probabilities for those in the first through fourth quartiles of mental HRQOL were 88%, 83%, 74%, and 77%, respectively.
FIGURE 1.
Kaplan-Meier survival estimates by physical and mental HRQOL quartiles. First quartile, 100th; second quartile, 75th; third quartile, 50th; fourth quartile, 25th.
Multivariate Analysis of HRQOL and Survival
We found that those in higher quartiles of physical HRQOL had lower relative hazards for death than those in the bottom quartile after controlling for mental HRQOL, age, gender, race, education, income, region, insurance status, HIV exposure group, CD4 count, stage of HIV disease, and use of HAART (Table 2). Specifically, those in the first quartile of physical HRQOL had only one quarter the hazard of death over the follow-up period compared to those in the fourth quartile. While the relative hazard of death declined monotonically with each higher quartile of physical HRQOL, the greatest drop occurred between the fourth and third quartiles. By contrast, in the same regression, there was no significant unique association of mental HRQOL with survival. For comparison, those on HAART had nearly one half the hazard of death of those on no antiretroviral medications, and those with CD4 count of less than 50 had 5.5 times the relative hazard of death compared to those with CD4 counts of 500 or greater. Other variables with significantly elevated relative hazards of death in the final model include older age, less than high school education, and exposure to HIV from other than male-to-male sexual contact, intravenous drug use, or heterosexual contact; variables associated with longer survival include lack of insurance and use of HAART. In a sensitivity analysis scoring the continuous HRQOL measures so that 1 point=1 standard deviation, the hazard ratio for physical HRQOL was 0.67 (95% confidence interval [CI], 0.53 to 0.85) and for mental HRQOL it was 0.99 (95% CI, 0.81 to 1.22).
Table 2.
The Multivariate Association of Physical and Mental HRQOL with Mortality in HCSUS
| Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|
| Physical HRQOL | |||
| PH 4th quartile | 1.00 | ||
| PH 3rd quartile | 0.61 | (0.49 to 0.76) | .00001 |
| PH 2nd quartile | 0.34 | (0.23 to 0.51) | .00001 |
| PH 1st quartile | 0.24 | (0.16 to 0.38) | .00001 |
| Mental HRQOL | |||
| MH 4th quartile | 1.00 | ||
| MH 3rd quartile | 0.95 | (0.70 to 1.29) | .75 |
| MH 2nd quartile | 1.24 | (0.77 to 1.98) | .37 |
| MH 1st quartile | 1.48 | (0.93 to 2.36) | .09 |
| Age | |||
| 18–34 | 1.00 | ||
| 35–49 | 0.99 | (0.78 to 1.25) | .94 |
| 50≤ | 1.38 | (0.97 to 1.96) | .08 |
| Gender | |||
| Male | 1.00 | ||
| Female | 0.98 | (0.75 to 1.29) | .90 |
| Race/ethnicity | |||
| White | 1.00 | ||
| Black | 1.13 | (0.89 to 1.43) | .32 |
| Hispanic | 0.90 | (0.65 to 1.24) | .52 |
| Other | 0.55 | (0.22 to 1.37) | .20 |
| Education | |||
| College degree | 1.00 | ||
| Some college | 1.21 | (0.91 to 1.59) | .18 |
| High school degree | 1.17 | (0.83 to 1.66) | .37 |
| No high school degree | 1.62 | (1.20 to 2.19) | .01 |
| Annual household income | |||
| > $25,000 | 1.00 | ||
| $10,001–$25,000 | 0.90 | (0.67 to 1.21) | .48 |
| $5,001–$10,000 | 0.93 | (0.62 to 1.39) | .72 |
| $0–$5,000 | 0.81 | (0.54 to 1.20) | .28 |
| Geographic region | |||
| West | 1.00 | ||
| Northeast | 0.73 | (0.51 to 1.05) | .09 |
| Midwest | 0.94 | (0.46 to 1.92) | .87 |
| South | 0.96 | (0.72 to 1.23) | .76 |
| Insurance status | |||
| Private/fee-for-service | 1.00 | ||
| Private HMO | 0.75 | (0.51 to 1.11) | .14 |
| Medicaid | 0.85 | (0.57 to 1.27) | .41 |
| Medicare | 1.11 | (0.79 to 1.56) | .53 |
| No insurance | 0.60 | (0.39 to 0.93) | .05 |
| HIV exposure | |||
| Male-to-male sexual contact | 1.00 | ||
| Injecting drug use | 1.15 | (0.81 to 1.63) | .44 |
| Heterosexual contact | 0.88 | (0.61 to 1.27) | .50 |
| Other mode | 1.59 | (1.08 to 2.33) | .05 |
| CD4 count | |||
| >500 | 1.00 | ||
| 200–499 | 1.08 | (0.40 to 2.92) | .87 |
| 50–199 | 2.44 | (0.98 to 6.09) | .06 |
| <50 | 5.95 | (2.66 to 13.3) | .001 |
| CDC stage of HIV infection | |||
| Asymptomatic | 1.00 | ||
| Symptomatic | 0.99 | (0.63 to 1.55) | .95 |
| AIDS | 1.53 | (0.95 to 2.45) | .08 |
| Antiretroviral medications | |||
| No antiretrovirals | 1.00 | ||
| 1 antiretroviral | 0.91 | (0.63 to 1.30) | .59 |
| 2 or more antiretrovirals | 0.72 | (0.52 to 0.98) | .05 |
| HAART | 0.54 | (0.35 to 0.83) | .01 |
HCSUS, HIV Cost and Services Utilization Study; HRQOL, health-related quality of life; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval.
To further understand the specific aspects of HRQOL most strongly associated with survival, we conducted additional analysis using the 8 original HRQOL measures (physical functioning, role functioning, freedom from bodily pain, general health, energy, social functioning, emotional well-being, and disability days) in place of the broader physical and mental HRQOL summary indices (Table 3). In these analyses, only the physical functioning and bodily pain scales were uniquely associated with survival, after controlling for covariates.
Table 3.
The Multivariate Association of the Original Eight HRQOL Measures with Mortality in HCSUS
| Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|
| Physical functioning | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 0.68 | (0.51 to 0.91) | .001 |
| 2nd quartile | 0.54 | (0.38 to 0.76) | .001 |
| 1st quartile | 0.48 | (0.33 to 0.71) | .01 |
| Role functioning* | |||
| Score 0–50 | 1.00 | ||
| Score 75–100 | 0.77 | (0.53 to 1.12) | .17 |
| Freedom from bodily pain | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 0.69 | (0.52 to 0.91) | .01 |
| 2nd quartile | 0.67 | (0.47 to 0.94) | .05 |
| 1st quartile | 0.65 | (0.48 to 0.89) | .01 |
| General health | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 1.03 | (0.82 to 1.29) | .82 |
| 2nd quartile | 0.68 | (0.51 to 0.91) | .01 |
| 1st quartile | 0.62 | (0.35 to 1.12) | .11 |
| Energy | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 1.00 | (0.82 to 1.22) | .99 |
| 2nd quartile | 1.36 | (0.99 to 1.88) | .06 |
| 1st quartile | 1.24 | (0.73 to 2.10) | .42 |
| Social functioning | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 0.84 | (0.69 to 1.03) | .09 |
| 2nd quartile | 1.03 | (0.72 to 1.47) | .88 |
| 1st quartile | 1.19 | (0.70 to 2.03) | .52 |
| Emotional well-being | |||
| 4th quartile | 1.00 | ||
| 3rd quartile | 1.09 | (0.86 to 1.38) | .49 |
| 2nd quartile | 1.06 | (0.66 to 1.71) | .80 |
| 1st quartile | 1.18 | (0.68 to 2.04) | .55 |
| Number of days in bed* | |||
| No days in bed | 1.00 | ||
| 1 or more days in bed | 0.95 | (0.76 to 1.19) | .63 |
| Age | |||
| 18–34 | 1.00 | ||
| 35–49 | 0.97 | (0.77 to 1.23) | .81 |
| 50≤ | 1.32 | (0.94 to 1.86) | .11 |
| Gender | |||
| Male | 1.00 | ||
| Female | 0.99 | (0.77 to 1.28) | .94 |
| Race/ethnicity | |||
| White | 1.00 | ||
| Black | 1.15 | (0.90 to 1.47) | .26 |
| Hispanic | 0.93 | (0.66 to 1.32) | .69 |
| Other | 0.60 | (0.25 to 1.43) | .25 |
| Education | |||
| College degree | 1.00 | ||
| Some college | 1.22 | (0.95 to 1.57) | .12 |
| High school degree | 1.17 | (0.83 to 1.65) | .37 |
| No high school degree | 1.58 | (1.19 to 2.10) | .01 |
| Annual household income | |||
| > $25,000 | 1.00 | ||
| $10,001–$25,000 | 0.91 | (0.66 to 1.26) | .58 |
| $5,001–$10,000 | 0.97 | (0.63 to 1.48) | .88 |
| $0–$5,000 | 0.82 | (0.55 to 1.23) | .34 |
| Geographic region | |||
| West | 1.00 | ||
| Northeast | 0.71 | (0.50 to 1.01) | .06 |
| Midwest | 0.93 | (0.46 to 1.85) | .83 |
| South | 0.96 | (0.73 to 1.25) | .75 |
| Insurance status | |||
| Private/fee-for-service | 1.00 | ||
| Private HMO | 0.80 | (0.52 to 1.22) | .29 |
| Medicaid | 0.84 | (0.55 to 1.29) | .43 |
| Medicare | 1.13 | (0.77 to 1.66) | .52 |
| No insurance | 0.61 | (0.39 to 0.95) | .05 |
| HIV exposure | |||
| Male-to-male sexual contact | 1.00 | ||
| Injecting drug use | 1.11 | (0.82 to 1.51) | .49 |
| Heterosexual contact | 0.86 | (0.60 to 1.24) | .43 |
| Other mode | 1.50 | (1.05 to 2.12) | .05 |
| CD4 count | |||
| >500 | 1.00 | ||
| 200–499 | 1.13 | (0.43 to 2.95) | .81 |
| 50–199 | 2.55 | (1.04 to 6.21) | .05 |
| <50 | 6.23 | (2.86 to 13.6) | .0001 |
| CDC stage of HIV infection | |||
| Asymptomatic | 1.00 | ||
| Symptomatic | 0.96 | (0.59 to 1.56) | .88 |
| AIDS | 1.42 | (0.87 to 2.32) | .16 |
| Antiretroviral medications | |||
| No antiretrovirals | 1.00 | ||
| 1 antiretroviral | 0.92 | (0.64 to 1.33) | .65 |
| 2 or more antiretrovirals | 0.73 | (0.54 to 1.00) | .05 |
| HAART | 0.57 | (0.38 to 0.86) | .01 |
Model includes physical functioning, role functioning, freedom from bodily pain, general health, energy, social functioning, emotional well-being, number of days in bed, age, gender, race/ethnicity, education, annual household income, geographic region, insurance status, HIV exposure, lowest CD4 count, CDC stage of HIV infection, and antiretroviral medications.
Role functioning and numbers of days in bed had to be dichotomized because of skewed distribution.
HCSUS, HIV Cost and Services Utilization Study; HRQOL, health-related quality of life; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval.
DISCUSSION
HIV infection is a lifelong condition. Because of recently developed treatments, the majority of people with the infection can live with it for long periods of time, and have productive, fulfilling lives. However, the threat of losing functional capacity and developing complications that affect functioning and well-being often persists. Measuring HRQOL can provide important information about the effects of disease progression and treatment that is not captured in laboratory measures of disease progression such as CD4 counts. While an increasing number of studies in the last decade have utilized HRQOL scales as outcome measures to quantify aspects of disease impact, these measures have rarely been used as early predictors of survival.
In this study, we found that the physical health dimension of HRQOL was a strong predictor of subsequent survival. Those in the first quartile of physical HRQOL had only one quarter the hazard of death compared to those in the fourth quartile, while the greatest drop occurred between the fourth and third quartiles. The finding of greatest mortality risk among those with the most severe physical impairment is consistent with findings of studies in HIV prior to the HAART era.18 Importantly, physical HRQOL added prognostic information over and above the standard clinical data we had available, namely CD4 count and stage of HIV infection based on CDC criteria. The magnitude of the effect of being in the highest quartile of physical HRQOL was comparable to that of using HAART versus no antiretrovirals in the population of American adults under care for HIV infection at the start of the HAART era. This finding suggests an important utility for measures of HRQOL in persons with HIV beyond that of a secondary outcome in clinical trials and observational studies. Measuring HRQOL in clinical settings may be useful for practicing health care providers trying to assess prognosis, and could lead to a search for prognostically important problems that might benefit from additional interventions.
Some studies provide support for the findings of this study. In several studies of patients with HIV prior to the HAART era, investigators have found a strong association between functional health status and survival.18,19 In addition, studies of elderly patients have shown that mortality after hospitalization can be predicted by functional health measures,20 and that long-term mortality is also predicted by functional health and general health measures in various populations.21–27 Previous studies have demonstrated that patient-reported HRQOL measurement in populations with HIV infection provides important information about health outcomes that are important to patients. These studies have demonstrated significant relationships in persons with HIV infection between HRQOL and clinical conditions, access to care, antiretroviral therapy, social support systems, psychological well-being, coping strategies, spiritual well-being, and psychiatric conditions.5,28–31 This study contributes importantly to the literature on assessing HRQOL in patients with HIV infection by also relating HRQOL (an intermediate outcome) with mortality (a distal outcome) in a national study.
Previous studies in other conditions have led to controversy about whether it is worth the time and effort to routinely assess HRQOL in clinical settings. Some authors ague that HRQOL measurement in clinical practice settings may improve quality of care through increasing the detection of patients' problems with daily functioning and well-being, guiding therapeutic management, and leading to improvements in patients' HRQOL.32,33 On the other hand, some recent literature has questioned the utility and cost-effectiveness of routine use.34,35 The present study supports the utility of HRQOL measurement in clinical settings.
The specific domains of HRQOL most associated with survival were physical functioning and bodily pain. This finding is supported by previous research indicating these two components as major indicators of physical HRQOL overall.13,36–38 In addition, this finding provides support for the usefulness of assessing physical HRQOL in clinical settings for at least two reasons. Recognizing the importance of physical functioning and bodily pain can help direct treatment to these problems and enable clinicians to improve HRQOL itself. Furthermore, these HRQOL deficits in particular may be reflective of underlying risk or illness severity that is not fully measured by lab values. Future studies should address the mechanisms underlying the link between HRQOL and mortality.
While unadjusted analyses suggested a relationship between mental HRQOL and survival similar to that with physical HRQOL, the relationship did not hold up in multivariate analysis that included both physical and mental HRQOL in the same model. The bivariate association between mental HRQOL and survival may have been due to the positive correlation between mental and physical HRQOL,39 only the latter of which was uniquely associated with survival. These varying relationships between different aspects of HRQOL and survival reinforce the importance of measuring HRQOL comprehensively in future research, to facilitate better understanding of these relationships. On the other hand, for clinical purposes, it appears that assessing physical HRQOL alone may be sufficient for prognosis of survival.
This study had several limitations. We had mortality information only through 1999, so we do not know whether the association between HRQOL and survival that we found would extend up to the present time. Furthermore, the sample includes only those with known HIV infection who were receiving medical care, outside of emergency rooms, jails, and prisons, so the findings may not generalize to those settings. We also lacked complete viral load data on the baseline sample. The strengths of the study include the national probability sample, the inclusion of clinical covariates, and multiple years of follow-up in the post-HAART era.
In summary, this study revealed several important findings. There was a strong association between physical HRQOL and survival, but not between mental HRQOL and survival, in the final model. The physical HRQOL effect persisted after controlling for several clinical variables, including CD4 count, CDC HIV stage, and HAART, most of which were strong predictors of survival in expected directions. The magnitude of the effect of physical HRQOL was comparable to that of the clinical and laboratory indicators, as well as treatment with HAART. These findings suggest that HRQOL measurement in populations with HIV infection provides important information about prognosis and risk stratification that goes beyond the clinical variables we included.
Acknowledgments
The HIV Cost and Services Utilization Study was conducted under cooperative agreement U-01HS08578 (M.F. Shapiro, PI; S.A. Bozzette, Co-PI) between RAND and the Agency for Health Research and Quality. Substantial additional funding for this cooperative agreement was provided by the Health Resources and Services Administration, the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Institutes of Health Office of Research on Minority Health through the National Institute of Dental Research. Additional support was provided by Merck and Company Incorporated, Glaxo-Wellcome Incorporated, the National Institute on Aging, and the Office of the Assistant Secretary for Planning and Evaluation in the U.S. Department of Health and Human Services. Dr. Hays was supported by a grant from the National Institute of Health (AI 28697). Drs. Cunningham and Hays also received partial support from the UCLA-Drew Project Export, National Institutes of Health, National Center on Minority Health and Health Disparities, (P20-MD00148-01), and the UCLA Center for Health Improvement in Minority Elders/Resource Centers for Minority Aging Research, National Institutes of Health, National Institute of Aging (AG-02-004).
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, December 2001 Year-end Edition. Vol. 13. Atlanta, GA: Centers for Disease Control and Prevention; 2001. pp. 1–48. [Google Scholar]
- 2.Hays RD, Cunningham WE, Sherbourne CD, et al. Health-related quality of life in patients with Human Immunodeficiency Virus Infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000;108:714–22. doi: 10.1016/s0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 3.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 4.Ganz PA, Schag CAC, Kahn B, Petersen L. Assessing the quality of life of HIV infected persons: clinical and descriptive information from studies with The Hopes. Psychol Health. 1994;9:93–110. [Google Scholar]
- 5.Globe DR, Hays RD, Cunningham WE. Associations of clinical parameters with health-related quality of life in hospitalized persons with HIV disease. AIDS Care. 1999;11:71–86. doi: 10.1080/09540129948216. [DOI] [PubMed] [Google Scholar]
- 6.Wu A, Hays RD, Kelly S, Malitz KF, Bozzette SA. Applications of the medical outcomes study health-related quality of life measures in HIV/AIDS. Qual Life Res. 1997;6:531–54. doi: 10.1023/a:1018460132567. [DOI] [PubMed] [Google Scholar]
- 7.Bozzette SA, Hays RD, Berry SH, Kanouse DE, Wu AW. Derivation and properties of a brief health status assessment instrument for use in HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:253–65. doi: 10.1097/00042560-199503010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chan KS, Revicki DA. Changes in surrogate laboratory markers, clinical endpoints, and health-related quality of life in patients infected with the human immunodeficiency virus. Eval Health Prof. 1998;21:265–81. doi: 10.1177/016327879802100207. [DOI] [PubMed] [Google Scholar]
- 9.Brechtl JR, Breitbart W, Galietta M, Krivo S, Rosenfeld B. The use of highly active antiretroviral therapy (HAART) in patients with advanced HIV infection: impact on medical, palliative care, and quality of life outcomes. J Pain Symptom Manage. 2001;21:41–51. doi: 10.1016/s0885-3924(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 10.Starace F, Ammassari A, Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(suppl 3):S136–S139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 11.Palella FJ, Delaney KM, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 12.Frankel MR, Shapiro MF, Duan N, et al. National probability samples in studies of low-prevalence diseases, part 2: designing and implementing the HIV cost and services utilization study sample. Health Serv Res. 1999;34:969–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Hays RD, Spritzer KL, McCaffrey DF, et al. The HIV Cost and Services Utilization Study (HCSUS) Measures of Health-related Quality of Life. Santa Monica, CA: RAND; 1998. DRU-1897-AHCPR. [Google Scholar]
- 14.Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services and Henry J. Kaiser Family Foundation. MMWR Recomm Rep. 1998;47(RR-5):43–82. [PubMed] [Google Scholar]
- 15.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in persons with HIV infection in the U.S. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Stata Corporation. Stata Reference Manual, V7.0. College Station, TX: Stata Corporation; 2000. [Google Scholar]
- 17.Bozzette SA, Berry SH, Duan N, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 18.Fleishman JA, Crystal S. Functional status transition and survival in HIV disease: evidence from the AIDS Costs and Service Utilization Survey. Med Care. 1998;36:533–43. doi: 10.1097/00005650-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson DL, Wu AW, Feinberg J. Outcomes Committee of the Adult AIDS Clinical Trials Group. Health-related quality of life predicts survival, cytomegalovirus disease, and study retention in clinical trial participants with advanced HIV disease. J Clin Epidemiol. 2003;56:874–9. doi: 10.1016/s0895-4356(03)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–93. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 21.Rumsfeld JS, MaWhinney S, McCarthy M, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281:1298–303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Community Health. 2000;54:123–9. doi: 10.1136/jech.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idler EL, Kasl S. Health perceptions and survival: do global evaluations of health status really predict mortality? J Gerontol. 1991;46:S55–S65. doi: 10.1093/geronj/46.2.s55. [DOI] [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 25.Konstam V, Salem D, Pouleur H, et al. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. SOLVD Investigations. Studies of Left Ventricular Dysfunction Investigators. Am J Cardiol. 1996;78:890–5. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 26.Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93:663–9. doi: 10.1016/0002-9343(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 27.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 28.Douaihy A, Singh N. Factors affecting quality of life in patients with HIV infection. AIDS Read. 2001;11:450–4. 460–1, 475. [PubMed] [Google Scholar]
- 29.O'Leary JF, Ganz PA, Wu AW, Coscarelli A, Petersen L. Toward a better understanding of health-related quality of life: a comparison of the Medical Outcomes Study HIV Health Survey (MOS-HIV) and the HIV Overview of Problems-Evaluation System (HOPES) J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:433–41. doi: 10.1097/00042560-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wilson IB, Cleary PD. Clinical predictors of declines in physical functioning in persons with AIDS: results of a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:343–9. doi: 10.1097/00042560-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham WE, Hays RD, Ettl MK, et al. The prospective effect of access to medical care on health-related quality-of-life outcomes in patients with symptomatic HIV disease. Med Care. 1998;36:295–306. doi: 10.1097/00005650-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Reemtsma K, Morgan M. Outcomes assessment: a primer. Bull Am Coll Surg. 1997;82:34–9. [PubMed] [Google Scholar]
- 33.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027–34. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 34.Kiebzak GM, Campbell M, Mauerhan DR. The SF-36 general health status survey documents the burden of osteoarthritis and the benefits of total joint arthroplasty: but why should we use it? Am J Manag Care. 2002;8:463–74. [PubMed] [Google Scholar]
- 35.Gilbody SM, House AO, Sheldon T. Routine administration of Health Related Quality of Life (HRQoL) and needs assessment instruments to improve psychological outcome—a systematic review. Psychol Med. 2002;32:1345–56. doi: 10.1017/s0033291702006001. [DOI] [PubMed] [Google Scholar]
- 36.Hays RD, Stewart AL. The structure of self-reported health in chronic disease patients. Psychol Assess. 1990;2:22–30. [Google Scholar]
- 37.Hays RD, Prince-Embury S, Chen H. R-36 HSI: RAND-36 Health Status Inventory. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 38.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 39.de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson's disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996;61:70–4. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]