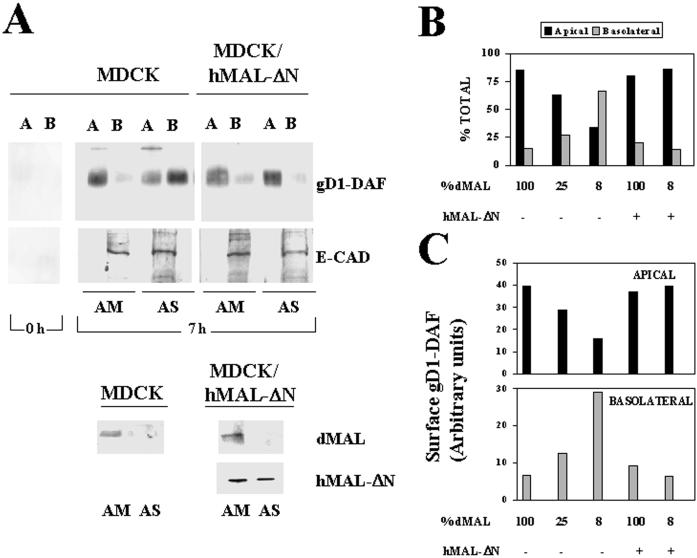
Figure 2.
MAL depletion in MDCK cells causes reduced transport of gD1-DAF to the apical surface and missorting to the basolateral membrane. (A) Normal MDCK cells or MDCK/hMAL-ΔN cells stably expressing gD1-DAF were transfected with AM or AS oligonucleotide, plated on 24-mm-diameter tissue culture inserts, and incubated at 37°C for 48 h. To study surface delivery of gD1-DAF, cells were repeatedly treated with sulfo-SHPP to quench the free amino groups on the cell surface from both the apical and basolateral faces of the insert. After incubation at 37°C for 0 or 7 h, the appearance of new free surface amino groups was monitored by domain-selective labeling with sulfo-NHS-biotin. After cell lysis, the extracts were immunoprecipitated with anti-gD1 antibodies, and newly surface delivered gD1-DAF was detected by immunoblotting with streptavidin-peroxidase. As a control, a similar analysis was done to detect surface delivery of E-cadherin, a basolateral protein of MDCK cells. The endogenous (dMAL) or exogenous (hMAL-ΔN) MAL levels in these cells were examined by immunoblot analysis with mAb 2E5 or 9E10, respectively. Note the efficiency of the treatment with sulfo-SHPP (0 h). (B and C) Quantitative analysis of the effect of MAL depletion on the polarized delivery of gD1-DAF. The intensity of the apical and basolateral signal corresponding to newly delivered surface gD1-DAF from experiments in which MAL was depleted at different extensions were quantified. The values obtained are represented as percentages of biotinylated gD1-DAF on the apical (black bars) or basolateral (gray bars) surface (B) or expressed in arbitrary units as apical (black bars) or basolateral (gray bars) absolute surface content of biotinylated gD1-DAF (C). The results of some representative experiments are shown. The effects observed in other experiments in which MAL was depleted to different extents (our unpublished results) were consistent with those shown.