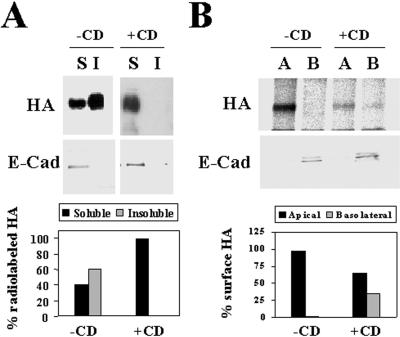
Figure 5.
Both insolubility and apical transport of HA in FRT cells are sensitive to methyl-β-cyclodextrin treatment. (A) FRT cells were pretreated or not in medium containing 25 μM compactin and 200 μM mevalonate for 48 h and infected with influenza virus. After 2 h of infection, the cultures pretreated with compactin and mevalonate were treated with 10 mM methyl-β-cyclodextrin (+CD) for 1 h, and then all cell cultures were labeled for 5 min with [35S]methionine/cysteine. After incubation in normal medium for 30 min at 20°C, cells were lysed with 1% Triton X-100 at 4°C, and the soluble (S) and insoluble (I) fractions were separated by centrifugation. Aliquots from these fractions were subjected to SDS-PAGE. The partition of newly synthesized HA was analyzed by autoradiography. As an internal control, the same samples were subjected to immunoblot analysis with anti-E-cadherin antibodies to show that E-cadherin was present exclusively in the soluble fractions. The bottom panel shows a quantitative analysis of the effect of the treatment with methyl-β-cyclodextrin on HA solubility. (B) FRT cells grown in filter culture inserts were pretreated or not with compactin plus mevalonate as in A and then infected with influenza virus. After 2 h, 10 mM methyl-β-cyclodextrin was added to both the apical and basolateral compartments of the pretreated cell cultures. After 1 h, cells were labeled for 5 min with [35S]methionine/cysteine and incubated in normal medium for 1 h at 37°C. Cells were surface biotinylated from either the apical or the basolateral compartment and lysed with 1% Triton X-100 plus 60 mM octyl-glucoside, and the lysates were immunoprecipitated with streptavidin-agarose. As a control, we observed that the total levels of newly synthesized radiolabeled HA were similar in control cells and in cells treated with methyl-β-cyclodextrin (our unpublished results). The apical or basolateral delivery of HA was monitored by autoradiography of the corresponding immunoprecipitates. As an internal control, the surface distribution of E-cadherin, a basolateral protein, was determined by immunoblot analysis of the streptavidin-agarose immunoprecipitates. The bottom panel shows a quantitative analysis of the effect of the treatment with methyl-β-cyclodextrin on the polarized delivery of HA to the cell surface.