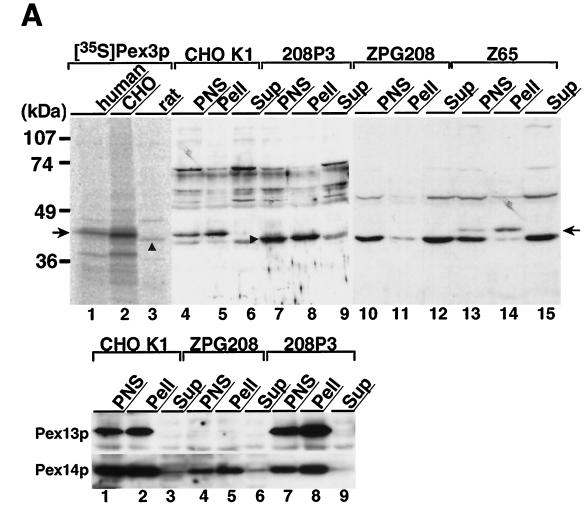
Figure 5.
Subcellular localization of Pex3p. (A) Upper panel, size comparison of in vitro transcription–translation products of human, Chinese hamster, and rat PEX3 cDNAs and subcellular localization of Pex3p in CHO cells. The [35S]Methionine- and [35S]cysteine-labeled in vitro transcription–translation product of each PEX3 and subcellular fractions of CHO cells were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Autoradiography for detection of 35S-labeled protein was exposed for 5 d; immunodetection was done with rabbit anti-Pex3p peptide antibody. Lanes 1–3, in vitro transcription–translation product (1 μl) of human, Chinese hamster, and rat PEX3, respectively; lanes 4–6, 7–9, 10–12, and 13–15, PNS, organellar, and cytosolic fractions (20 μg each) of CHO-K1 and 208P3, a stable RnPEX3 transfectant of ZPG208, ZPG208, and a pex2 mutant, Z65, a typical peroxisome membrane remnant-positive CHO mutant (Tsukamoto et al., 1991; Shimozawa et al., 1992; Tsukamoto et al., 1994b), respectively. A composite of two separate experiments is represented. The arrow indicates the migration of human and Chinese hamster Pex3p; the arrowhead shows rat Pex3p. Lower panel, expression level of Pex13p and Pex14p in pex3 mutant ZPG208. Subcellular fractions (30 μg each) of CHO-K1 and pex3 mutant ZPG208 and 208P3 were analyzed by immunoblot using antibodies to Pex13p and Pex14p. Exposure for detection of Pex13p was several times longer than that for Pex14p. (B) Isopycnic subcellular fractionation. A light mitochondrial fraction of rat liver was fractionated by isopycnic sucrose density gradient ultracentrifugation. The gradient was collected into 36 tubes. After marker enzyme and protein assays, an equal volume (15 μl, 2 μl for detection of catalase) of odd-numbered fractions plus fraction 24 was analyzed by immunoblot. Upper panel, distribution of marker enzymes: catalase for peroxisomes; glutamate dehydrogenase, mitochondria; esterase, microsomes; and N-acetyl-β-glucosaminidase, lysosomes. Lower panel, immunoblots with antisera against Pex3p, Pex14p, PMP70, catalase, and AOx. Arrows, AOx components A–C. Results are presented in the direction of lower to higher density of sucrose, from left to right.