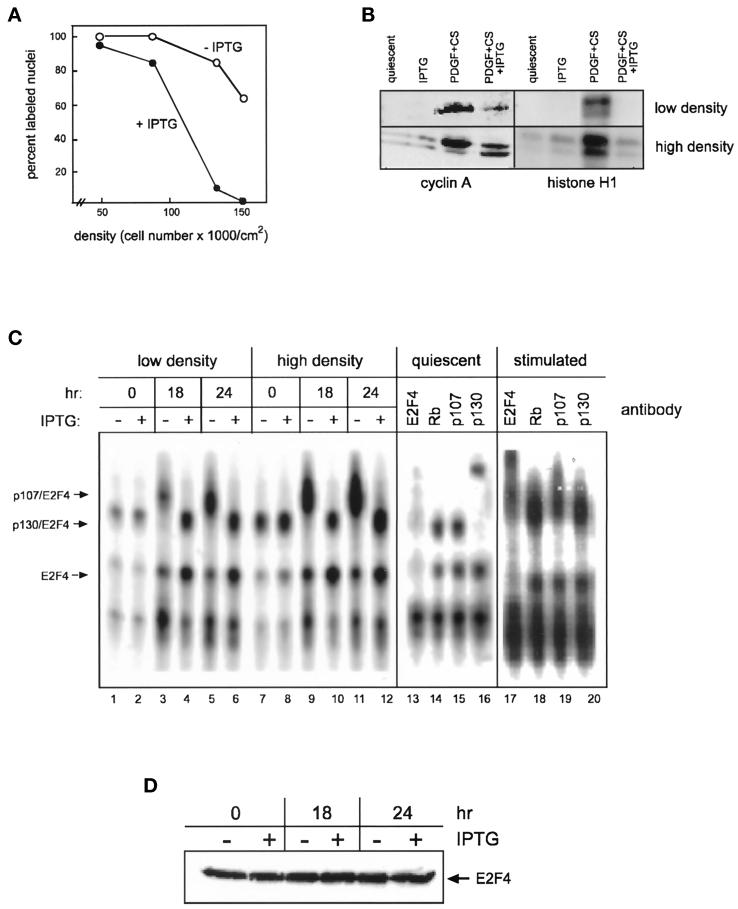
Figure 6.
Effect of cell density on the growth of cells expressing elevated amounts of p27kip1. (A) p27-47 cells were seeded at a density of 6000 cells/cm2 in 100-mm plates and grown to confluency in medium containing various concentrations of serum; cell number was determined. Replicate cultures were pretreated overnight with (closed circles) or without (open circles) 1 mM IPTG and subsequently refed with medium supplemented with 20 ng/ml PDGF, 10% PPP, 1 mM IPTG, and 5 μCi/ml [3H]thymidine. Cells were harvested 48 h later, and the percentage of cells incorporating [3H]thymidine was determined by autoradiography. (B) p27-47 cells were arrested at low or high density and pretreated overnight with 1 mM IPTG. After pretreatment, cultures either were harvested (quiescent, IPTG) or received fresh medium containing 10 ng/ml PDGF and 10% calf serum (CS) with or without IPTG for 24 h. Cell extracts were prepared and immunoblotted with cyclin A antibody(left panels) or immunoprecipitated with cyclin A antibody and assayed for kinase activity (right panels). (C) p27-47 cultures were arrested and pretreated with IPTG as in B. Lanes 1–12, cells were stimulated with 10 ng/ml PDGF and 10% calf serum plus or minus IPTG for 18 or 24 h, and cell extracts were assayed for E2F DNA binding activity by EMSA. Lanes 13–16, extracts of low-density quiescent IPTG-pretreated cells were incubated with the indicated antibodies before addition of the radiolabeled probe. Lanes 17–20, extracts of high-density cells stimulated with PDGF plus serum in the absence of IPTG were incubated with the indicated antibodies before addition of the radiolabeled probe. (D) High-density cultures were pretreated overnight with 1 mM IPTG and stimulated with 10 ng/ml PDGF and 10% calf serum with or without 1 mM IPTG for 18 or 24 h. Cell extracts (175 μg) were immunoblotted with antibody to E2F4.