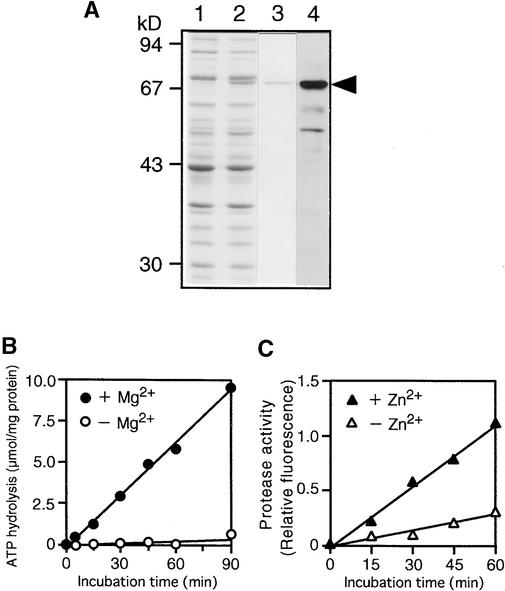
Figure 3.
Expression and Enzymatic Activity of the Recombinant DS9 Protein.
(A) Expression and purification of the recombinant HisDS9 protein from E. coli cells. Total cellular extracts prepared from cells not treated with IPTG (lane 1) and IPTG-treated cells (lane 2) from E. coli were separated by electrophoresis on a 10% SDS–polyacrylamide gel; the gel was stained with Coomassie Brilliant Blue R 250. The recombinant HisDS9 protein (arrowhead) was purified by affinity chromatography (lane 3) and subjected to protein gel blot analysis (lane 4) with antibodies raised against DS9ΔN. Size markers are indicated at left in kilodaltons.
(B) Mg2+-dependent ATP-hydrolyzing activity in the recombinant HisDS9 protein. Reactions were performed in the presence (closed circles) or absence (open circles) of 5 mM MgCl2.
(C) Zn2+-stimulated casein-hydrolyzing activity in the recombinant HisDS9 protein. Fluorescein isothiocyanate–labeled casein was used as the substrate. Reactions were performed in the presence (closed triangles) or absence (open triangles) of 12.5 μM zinc acetate.