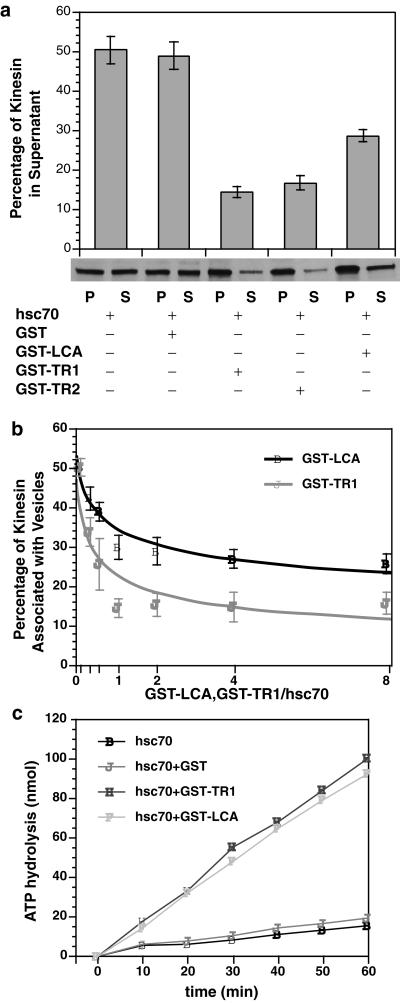
Figure 6.
Fusion proteins containing KLC or KLC tandem repeats inhibited the ability of hsc70 to release kinesin from vesicles. (a) Hsc70-mediated release of kinesin from V2 vesicle fractions was assayed as described above. Four different constructs were generated: GST, GST-LCA, GST-TR1, and GST-TR2. At a molar ratio of 1:1 for fusion protein:hsc70, GST-LCA, GST-TR1, and GST-TR2 constructs inhibited release of kinesin by hsc70. In contrast, GST alone was inactive in these assays. Constructs containing only KLC tandem repeat domains (TR1 and TR2) were more effective than ones also containing other KLC domains (GST-LCA). Recombinant KLC and tandem repeat fusion proteins differ in their relative efficacy to inhibit hsc70-mediated release of kinesin. (b) The lesser ability of GST-LCA to inhibit hsc70-mediated release relative to GST-TR1/TR2 might reflect differences in the amount of active protein or an effect of domains in KLC other than the tandem repeats. If the amount of active protein differs, increased molar ratios of KLC to hsc70 should reduce observed differences. However, at all molar ratios tested, GST-TR1 was more effective at inhibiting kinesin release by hsc70 than GST-LCA. The effects of GST-LCA appeared to plateau at ∼30%. (c) To determine whether GST-LCA and GST-TR1 differed in their interactions with hsc70, the ability of different constructs to activate hsc70 ATPase activity was evaluated. Both GST-LCA and GST-TR1 stimulated hsc70 ATPase activity to a similar extent, indicating a comparable ability to bind and activate hsc70 ATPase. GST alone was inactive. Therefore, differences in the ability of LCA and TR1 fusion proteins to inhibit hsc70-mediated release of kinesin from vesicles were not due to differences in their ability to interact with hsc70, so KLC domains outside the tandem repeats must affect the ability of hsc70 to release kinesin from MBOs. Error bars represent ±SEM; n = 3 experiments unless otherwise specified.