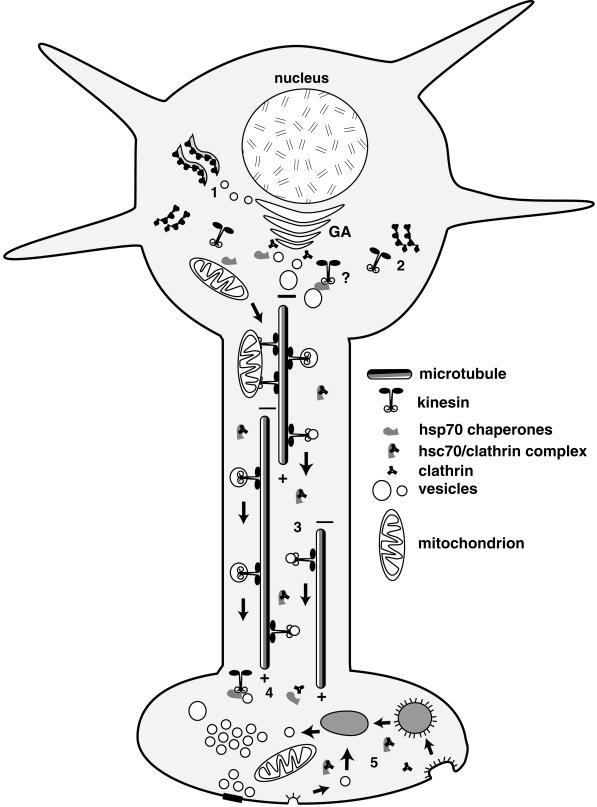
Figure 8.
Model for hsc70 regulation of kinesin-mediated axonal transport. The molecular chaperone hsc70 may regulate kinesin binding in both the cell body and in subcellular domains targeted for delivery of material in fast axonal transport. To package materials for movement in fast axonal transport, membrane proteins must be synthesized on rough endoplasmic reticulum (1) and packaged in the Golgi apparatus (GA). Kinesin is not associated with Golgi membranes (Pfister et al., 1989b; Leopold et al., 1992) but is enriched on transport vesicles near the trans-Golgi face (see, for example, Figures 1 and 7). Kinesin motors are synthesized on free polysomes (2) and must be added to transport vesicles post-Golgi. This pathway has not been characterized, but the role of chaperones in delivering proteins synthesized on free polysomes to organelles such as the mitochondrion and the enrichment of hsc70 near the trans-Golgi face suggest a role for hsc70 adding kinesin to MBOs in the cell body. Vesicles with kinesin motors are moved in fast axonal transport toward the plus ends of microtubules (3) away from the cell body. In the axon, hsc70 is complexed with cytoplasmic proteins such as clathrin (Black et al., 1991). Upon reaching a presynaptic terminal, kinesin is removed from MBO surfaces (4) by available hsc70 that is released from cytoplasmic complexes with clathrin or other proteins. This frees clathrin to play a role in vesicle recycling (5), in which hsc70 may also be important for removal of clathrin coats from coated vesicles that recycle synaptic vesicle components or repackage membrane proteins for return to the cell body by retrograde transport mediated by dynein. Cytoplasmic kinesin appears to be rapidly degraded in terminal regions after release from membranes, because it is not enriched in terminal regions or returned by retrograde transport (Li et al., 1999).