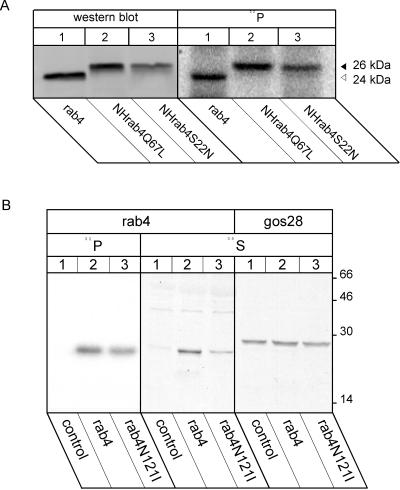
Figure 1.
Phosphorylation of rab4 mutants in vivo. Mitotic cells were labeled for 2 h with 175 μCi/ml 32P ortho phosphate and lysed in 1% Triton X-100 in PBS. Rab4 was immunoprecipitated with a rab4 antibody or with the monoclonal NH antibody from the cell lines expressing the GDP -bound or GTPase hydrolysis deficient mutants. Immunoprecipitates were resolved by SDS-PAGE on 12.5% minigels and analyzed by phosphorimaging. Aliquots of unlabeled mitotic cells were subjected to Western blotting using rab4 antibodies, and results were quantitated using Imagequant (A). To investigate phosphorylation of the nucleotide free transition state, CHO cells expressing rab4N121I were labeled with 32P ortho phosphate and immunoprecipitated. Expression of this rab4 mutant was assayed by immunoprecipitation from detergent lysates that were prepared after labeling mitotic cells with 200 μCi/ml 35S Trans label for 45 min. Immunoprecipitation of the cis Golgi SNARE GOS28 served as internal control for the expression level of rab4 (B). Normalization of the 32P signals with respect to rab4 expression in the four cell lines revealed that phosphorylation was independent of its guanine nucleotide status.