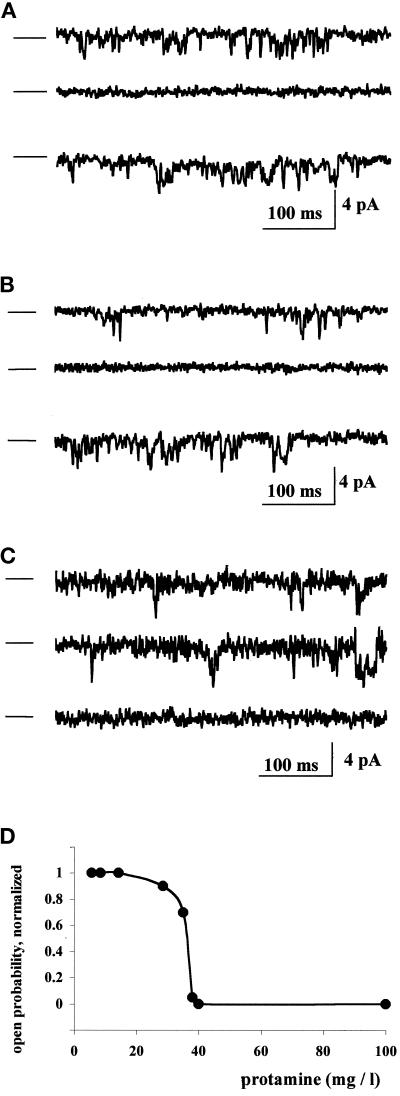
Figure 2.
RyR1 from rabbit skeletal muscle sarcoplasmic reticulum vesicles is inhibited by protamine sulfate (clupeine). Single-channel activity was recorded in the presence of 0.3 μM free calcium on the cytoplasmic side of the RyR. Zero current levels are indicated by the bar at the left of each trace, and openings are shown as downward deflections. Panels A, B, and C show recordings from three different experiments. The top trace of each panel shows channel activity before drug application. In A and B, the addition of 40 μg/ml protamine to the cytosolic side of the channel leads to inactivation of the RyR and complete loss of channel activity (middle trace). Removal of protamine by perfusion with buffer (A) or by the addition of 40 μg/ml heparin to the cytoplasmic side of the channel (B) restores channel activity (bottom trace). The addition of protamine cross-linked with beaded agarose (activity equivalent to 40 μg/ml protamine) to an active RyR channel does not change the open probability (C, middle trace); further addition of free protamine (40 μg/ml), however, blocks channel activity (C, bottom trace). In D, the effects of different protamine concentrations on RyR channel activity in the presence of 0.3 μM free calcium on the cytoplasmic side of the RyR were tested in three independent experiments. Protamine concentrations of <15 μg/ml did not affect active RyR channels, whereas 20–35 μg/ml protamine reduced channel open probability slightly, and concentrations of 38 μg/ml or greater inhibited channel activity substantially. A protamine concentration of >40 μg/ml completely inhibited RyRs. Protamine addition never increased channel activity. Half-maximal inhibition was obtained at 37 ± 1 μg/ml (n = 3), and a Hill coefficient >4 was observed.