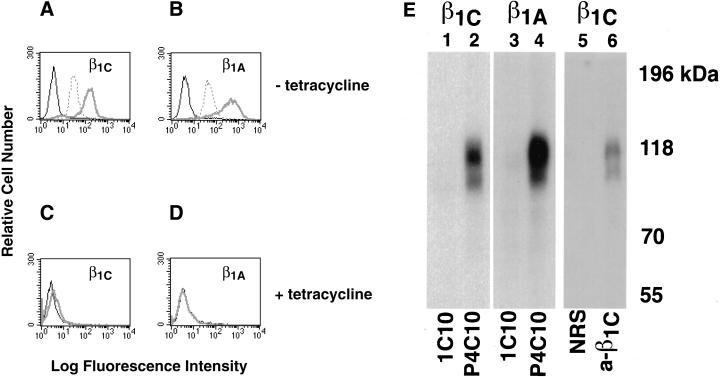
Figure 1.
Surface expression of β1C and β1A in CHO cells. (A–D) β1C or β1A CHO stable cell lines were cultured for 48 h either in the absence (A and B) or in the presence (C and D) of 1 μg/ml tetracycline and analyzed by FACS with TS2/16 mAb to human β1 integrin, 7E2 mAb to hamster β1 integrin, or 12CA5 as a negative control, followed by FITC goat anti-mouse immunoglobulin G. Fluorescence intensity is expressed in arbitrary units. FACS analysis of a representative clone for each β1 variant is shown. Thick gray line, TS2/16; dotted line, 7E2; thin black line, 12CA5. (E) CHO stable cell lines were cultured as in A and B and surface-labeled with Na 125I; exogenous β1 integrins were immunoprecipitated with P4C10 mAb to human β1 integrin (lanes 2 and 4). The immunoprecipitated material was then eluted from protein A–Sepharose with 50 mM Tris-HCl, pH 7.5, 2% SDS and boiled for 5 min. The immunocomplexes were then reprecipitated with rabbit antiserum to the β1C cytoplasmic domain (lane 6) and separated on 7.5% SDS-PAGE. mAb 1C10 (lanes 1 and 3) or normal rabbit serum (lane 5) were used as negative controls. Lanes 1, 2, 5, and 6, β1C CHO; lanes 3 and 4, β1A CHO. Proteins were visualized by autoradiography. Prestained marker proteins (in kilodaltons) are shown.