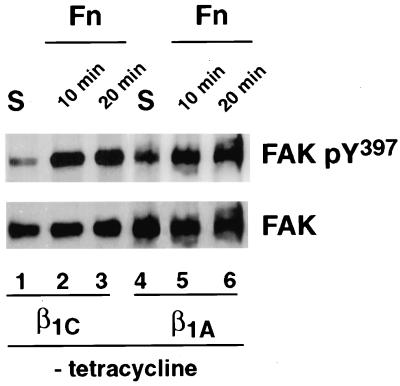
Figure 4.
Expression of β1C integrin does not affect FAK activation. β1C or β1A CHO stable cell lines were cultured for 48 h in the absence of tetracycline and serum-starved during the last 24 h of the 48-h culture. The cells were detached and either held in suspension (S; lanes 1 and 4) or plated on tissue culture plates coated with fibronectin (Fn; lanes 2, 3, 5, and 6) for either 10 or 20 min at 37°C. FAK was immunoprecipitated from 500 μg of total cell lysate with 0.5 μg of affinity-purified antibody to FAK, and its phosphorylation was analyzed by immunoblotting with 0.2 μg/ml phospho-specific antibody to Tyr397. FAK protein levels were analyzed with 0.1 μg/ml affinity-purified antibody to FAK, and proteins were visualized by enhanced chemiluminescence. The experiments were repeated twice with consistent results.