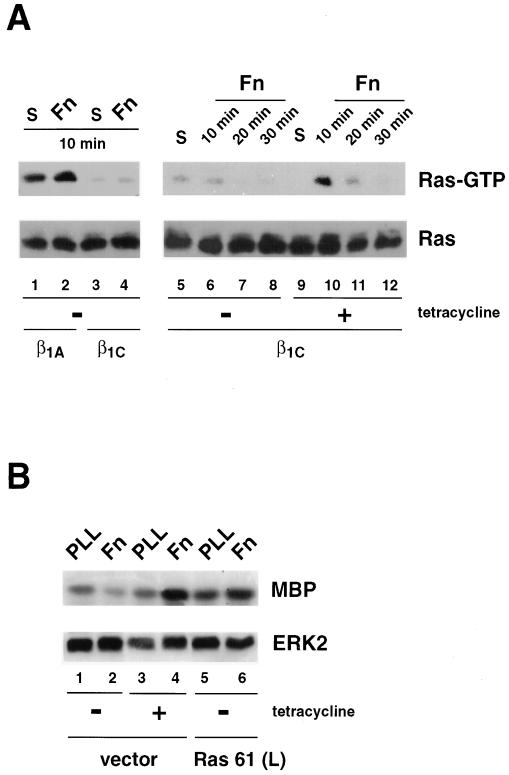
Figure 7.
β1C prevents Ras activation stimulated by fibronectin. (A) β1C or β1A CHO stable cell lines were cultured for 48 h either in the absence (lanes 1–8) or in the presence (lanes 9–12) of 1 μg/ml tetracycline and serum-starved during the last 24 h of the 48-h culture. The cells were detached and either held in suspension (S; lanes 1, 3, 5, and 9) or seeded on tissue culture plates coated with fibronectin (Fn; lanes 2, 4, 6–8, and 10–12) for 10 min (lanes 1–6, 9, and 10), 20 min (lanes 7 and 11), or 30 min (lanes 8 and 12) at 37°C. Cells were lysed, and Ras activation was analyzed by affinity precipitation with GST-RBD (top panels). Ras proteins were detected by immunoblotting with 2 μg/ml mAb to Ras (bottom panels). (B) β1C CHO stable cell lines were transiently transfected with constitutively activated Ras [Ras 61 (L); lanes 5 and 6] or vector alone (vector; lanes 1–4). Cells were cultured for 48 h either in the absence (lanes 1, 2, 5, and 6) or in the presence (lanes 3 and 4) of 1 μg/ml tetracycline and starved during the last 24 h of the 48-h culture. Transfected cells were then detached and plated on dishes coated with either poly-l-lysine (PLL; lanes 1, 3, and 5) or fibronectin (Fn; lanes 2, 4, and 6) for 10 min at 37°C. ERK2 in vitro kinase activity (top panel) and expression (bottom panel) were analyzed as described for Figure 6. In A and B (bottom panels), proteins were visualized by enhanced chemiluminescence. The experiments were repeated twice with consistent results.