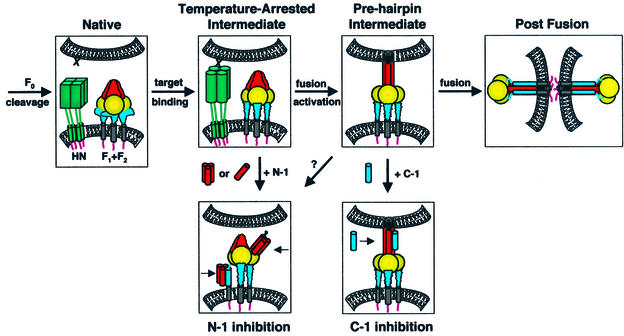
Fig. 8. Model of the paramyxovirus fusion mechanism. Four steps in SV5 F-mediated fusion are F0 cleavage, HN binding to target cells, activation of F for fusion and F-mediated fusion. The N-1 peptide inhibits a temperature-arrested intermediate that forms after the binding of HN (but not uncleaved HA) to target cells, consistent with the binding of HN to its receptor sialic acid stimulating a conformational change in F. The N-1 binding site becomes unavailable as a function of time after thermal activation. Plausible binding modes of N-1 to F regions are binding HRB as trimers or HRA as monomers/dimers. C-1 binds and inhibits a pre-hairpin intermediate of F that forms after thermal activation and remains until a point directly preceding membrane merger, as probed by LPC arrest. The data presented here suggest that six-helix bundle formation and membrane fusion are concurrent events. F domains: fusion peptide (black); HRA/N-1 (red); intervening domain (gold); HRB/C-1 (blue); TM domain (gray). As F2 and F1 are structurally integral parts of the F protein, these subunits are not shown separately.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.