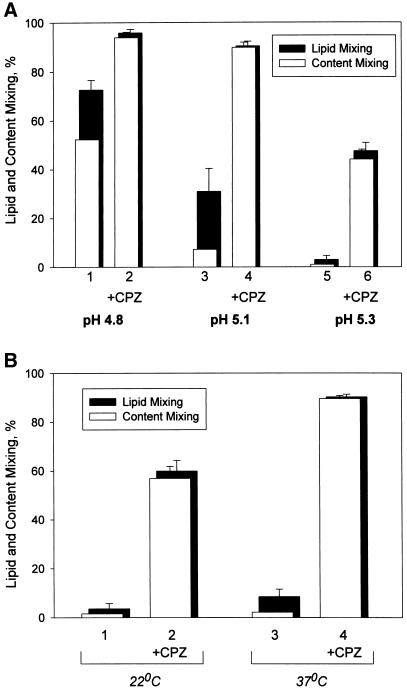
Figure 1.
Fusion intermediate detected at decreased numbers of low pH–activated HA molecules allows neither content nor lipid mixing before CPZ application. (A) Decreasing the number of low pH-activated HA molecules shifts the fusion phenotype from complete fusion to UH and then to RH. Fusion of X31 HA-cells with bound PKH26- and carboxyfluorescein-labeled RBC was triggered at 37°C by a 2-min application of pH 4.8 (bars 1 and 2), pH 5.1 (bars 3 and 4), and pH 5.3 (bars 5 and 6). As determined by CELISA, these low pH pulses activated 97 ± 0.2%, 20 ± 0.1%, and 6.4 ± 0.7% of HA for pH 4.8, 5.1, and 5.3, respectively (mean ± SE of quadruplicate determinations). In the experiments indicated by bars 2, 4, and 6, immediately after the end of a low pH pulse cells were exposed to a 1-min application of 0.25 mM CPZ. The extent of fusion was assayed by fluorescence microscopy at neutral pH 20 min after the end of low pH application as lipid dye (PKH26; closed bars) and aqueous dye (carboxyfluorescein; open bars) redistribution. Bars indicate mean ± SE, n > 3. The percentage of RBC/HA-cell contacts containing RH intermediates at different pH can be estimated as the difference between the extents of lipid mixing with and without CPZ application. (B) RH formation at 22°C (bars 1 and 2) and 37°C (bars 3 and 4). Lipid and content mixing (closed and open bars) in fusion of X31 HA-cells with bound PKH26- and carboxyfluorescein-labeled RBC, triggered by a 5-min pulse of pH 5.3, were assayed as in A. In the experiments indicated by bars 2 and 4, the low pH pulse was followed by a 1-min pulse of 0.25 mM CPZ applied at 22°C.