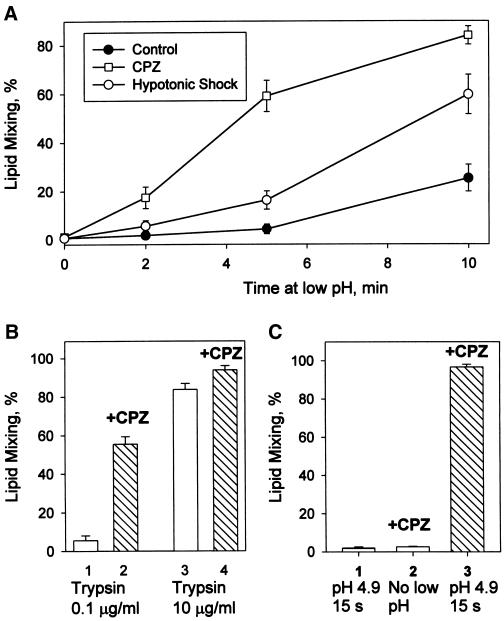
Figure 2.
RH detection at suboptimal fusion conditions such as moderately acidic pH (A), low surface density of trypsin-cleaved fusion-competent HA (B), and short pH applications (C). (A) At moderately acidic pH, an increase in the number of RH intermediates outpaces an increase in lipid mixing. Fusion of Japan HA-cells with bound RBC was triggered at room temperature by applying pH 5.35 for different times (2, 5, and 10 min). Closed circles represent the final extents of lipid mixing observed after these pH pulses; squares and open circles represent final lipid mixing extents observed when low pH pulses were followed immediately by CPZ (0.5 mM, 1 min) or HOS, respectively. Points indicate mean ± SE, n > 3. (B) RH formation at 37°C and pH 4.9 for cells with decreased numbers of trypsin-cleaved HA molecules. Lipid mixing extents observed for X31 HA-cells pretreated with 0.5 U/ml neuraminidase and either 0.1 μg/ml (bars 1 and 2) or 10 μg/ml (bars 3 and 4) trypsin (10 min, 22°C). Fusion was triggered by a 2-min pulse of pH 4.9 followed (bars 2 and 4) or not followed (bars 1 and 3) by a CPZ pulse (0.25 mM, 1 min). Bars indicate mean ± SE, n > 3. (C) Fast formation of RH intermediates after treating X31 HA-cells with bound RBC with a 15-s pulse of pH 4.9 at 37°C. RH was detected with a 20-s pulse of 0.5 mM CPZ applied immediately after the end of low pH application. Bar 1, lipid mixing extent observed in the control experiment with no CPZ pulse; bar 2, the control experiment in which CPZ was applied to RBC/HA-cell pairs, which were not treated with low pH; bar 3, the cells were treated with low pH and, already at neutral pH, with CPZ. Bars indicate mean ± SE, n > 3.