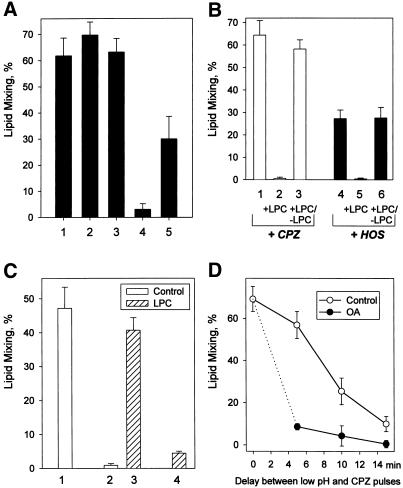
Figure 4.
Effects of 4°C and membrane lipid composition on the properties of transient RH intermediates. (A) Dissociation of RH intermediates is blocked at 4°C. Fusion of X31 HA-cells with bound RBC was triggered by a 2-min pulse of pH 5.2 at 22°C. Bars 1 and 4, a low pH pulse was followed by a 1-min pulse of 0.5 mM CPZ applied immediately after a low pH pulse (1) or 20 min later (4). Bars 2 and 3, immediately after the end of a low pH pulse, the temperature was decreased to 4°C. After 20-min (2) or 90-min (3) incubation at 4°C, the temperature was returned to 22°C, and a CPZ pulse (0.5 mM, 1 min) was applied. Lipid mixing extents were determined at least 20 min after a CPZ pulse. As for bar 2, after the end of a low pH pulse the cells were incubated at 4°C for 20 min. Then, still at 4°C, a 1-min pulse of CPZ was applied. After incubating cells for another 10 min at 4°C, the temperature was increased to 22°C, and lipid mixing extents were assayed. Without CPZ application, lipid mixing observed after this low pH treatment at 22°C was only 3 ± 1.4%. Bars indicate mean ± SE, n > 3. (B) LPC reversibly suppresses RH intermediates as detected with CPZ (bars 1–3) or HOS (bars 4–6). Fusion of X31 HA-cells with bound RBC was triggered at 37°C by a 2-min pulse of pH 5.3. Immediately after the pulse, the cells were treated with either CPZ (0.25 mM, 1 min) or HOS (bars 1 and 4, respectively). In the experiments indicated by bars 2, 3, 5, and 6, after the pulse low pH medium was replaced with LPC-supplemented PBS (pH 7.4, 150 μM LPC). One minute later, a CPZ pulse (bar 2) or HOS (bar 5) was applied, still in the presence of LPC. In the experiments indicated by bars 3 and 6, LPC was washed out before CPZ (bar 3) and HOS (bar 6) application. Bars represent the mean extents of lipid mixing ± SE, n > 3. No measurable lipid mixing was observed after this low pH treatment with neither CPZ nor HOS applied. (C) LPC blocks inactivation of the HA machinery responsible for the formation of transient RH intermediates. Fusion of Japan HA-cells with bound RBC was triggered at room temperature by a 2-min pulse of pH 5.3. Bar 1, low pH pulse followed by a CPZ pulse (0.5 mM, 1 min). Bar 2, low pH pulse followed by a 10-min incubation at neutral pH and then a CPZ pulse. Bar 3, low pH pulse followed by a 10-min incubation atneutral pH in the presence of 80 μM LPC; LPC was then washed out, and the cells were treated with a CPZ pulse (0.5 mM, 1 min). Bar 4, low pH pulse followed by a 10-min incubation at neutral pH, still in the presence of LPC; LPC was then washed out, and the cells were incubated for another 10 min in the absence of LPC and finally treated with a CPZ pulse. Bars indicate mean ± SE, n > 3. (D) RH dissociation proceeds faster in the presence of oleic acid (OA). Fusion of Japan HA-cells with bound RBC was triggered by a 1-min pulse of pH 5.3 at room temperature. After the pulse, low pH medium was replaced with PBS (pH 7.4) supplemented (●) or not (○) with 10 μM oleic acid. Then, after different time intervals, the cells were treated with CPZ (0.25 mM, 1 min). Bars represent the mean extents of lipid mixing ± SE, n > 3.