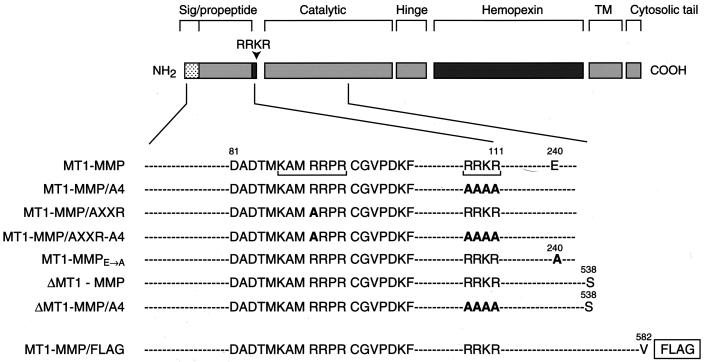
Figure 1.
A scheme of MT1-MMP mutants. MT1-MMP domains are depicted as shaded boxes. Amino acid substitutions were inserted either into the 108RRKR and 86KAMRRPR domains (each bracketed) alone as 108RRKR → AAAA (MT1-MMP/A4) or 86KAMRRPR → KAMARPR (MT1-MMP/AXXR) or into both domains as an MT1-MMP/AXXR-A4 mutant. Additional mutations included a 240E → A substitution in the catalytic domain to create an enzymically inactive mutant of MT1-MMP, a soluble transmembrane-deletion mutant with the C terminus truncated at S538 (ΔMT1-MMP), a soluble mutant with an RRKR → AAAA substitution (ΔMT1-MMP/A4), or an epitope-tagged variant of MT1-MMP with FLAG inserted at the C terminus of the wild-type proteinase (MT1-MMP/FLAG).