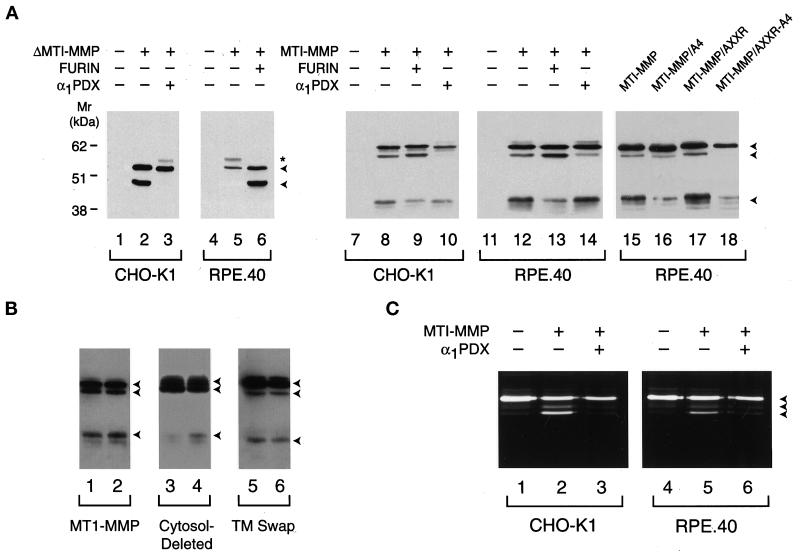
Figure 5.
MT1-MMP processing and activation in CHO-K1 and RPE.40 cells. (A) Processing of soluble and full-length MT1-MMP. CHO-K1 (lanes 1–3) or RPE.40 (lanes 4–6) cells were transfected with a control expression vector (lanes 1 and 4), ΔMT1-MMP (lanes 2 and 5), ΔMT1-MMP and α1PDX (lane 3), or ΔMT1-MMP and furin (lane 6) expression vectors. Cell-free supernatants were subjected to immunoblot analysis with anti-hemopexin domain–specific polyclonal antisera. The arrowheads to the right of lane 6 mark the positions of the pro and mature forms of ΔMT1-MMP, whereas the asterisk marks the position of a minor glycosylated form of proΔMT1-MMP as determined by tunicamycin sensitivity (our unpublished results). In lanes 8–10 and 12–14, Western blotting of Triton X-114 extracts was performed on CHO-K1 or RPE.40 cells expressing full-length MT1-MMP that had been cotransfected with a control expression vector (lanes 8 and 12), a furin expression vector (lanes 9 and 13), or an α1PDX expression vector (lanes 10 and 14). Immunoblots of CHO-K1 or RPE.40 cells transfected with control expression vectors are shown in lanes 7 and 11. In lanes 15–18, RPE.40 cells were transfected with expression vectors for MT1-MMP (lane 15), MT1-MMP/A4 (lane 16), MT1-MMP/AXXR (lane 17), or MT1-MMP/AXXR-A4 (lane 18). (B) MT1-MMP processing in CHO-K1 and RPE.40 cells. Western blotting was performed on CHO-K1 and RPE.40 cell extracts that had been transfected with full-length MT1-MMP (lanes 1 and 2, respectively), cytosolic domain–deleted MT1-MMP (lanes 3 and 4), or a chimeric MT1-MMP variant containing the interleukin 2 receptor transmembrane domain and cytosolic tail (TM swap; lanes 5 and 6). The arrowheads to the right mark the positions of the pro, mature, and truncated forms of MT1-MMP. (C) Processing of progelatinase A by CHO-K1 or RPE.40 cells. CHO-K1 or RPE.40 cells transfected with control expression vectors (lanes 1 and 4), cotransfected with MT1-MMP and control expression vectors (lanes 2 and 5), or cotransfected with MT1-MMP and α1PDX expression vectors (lanes 3 and 6) were incubated with progelatinase A for 16 h, and the supernatants were analyzed by gelatin zymography. The pro, intermediate, and mature forms of gelatinase A are marked by the arrowheads to the right of lane 6.