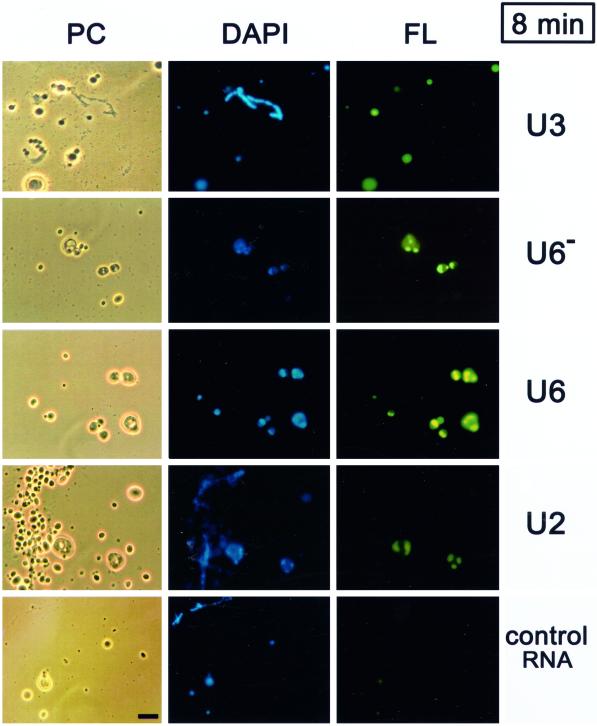
Figure 1.
Short term nucleolar localization of U6 snRNA. Fluorescein-labeled U3 snoRNA, U6 snRNA, U2 snRNA, or a 40-nt control RNA were injected into the nuclei of Xenopus laevis oocytes. After 8 min, nuclear spreads were prepared and analyzed by phase contrast (PC) or by fluorescence microscopy (FL green). Nucleoli can be distinguished from other nonchromosomal nuclear bodies because only the nucleoli contain DNA which is visualized by staining (DAPI blue). U3 snoRNA localizes to nucleoli only modestly at this time point, whereas U6 snRNA shows strong nucleolar signals in the dense fibrillar component that surrounds the DAPI-positive rDNA. U6 snRNA that does not carry a stabilizing 5′ cap (U6−) shows a higher variability of signals than transcripts with a 5′ cap (U6), but generally localizes well to nucleoli. U2 snRNA in an equimolar amount to U6 also stains nucleoli, although weakly, whereas the 40-nt control RNA even at five times the molar amount does not stain nucleoli. A lampbrush chromosome is visible in the U3 panel and also in the U2 panel (see PC and blue stain in DAPI) where it is coated with B-snurposomes, which are DAPI-negative. The snurposomes are not labeled by any of the injected RNAs after 8 min. Bar, 10 μm.