Figure 3.
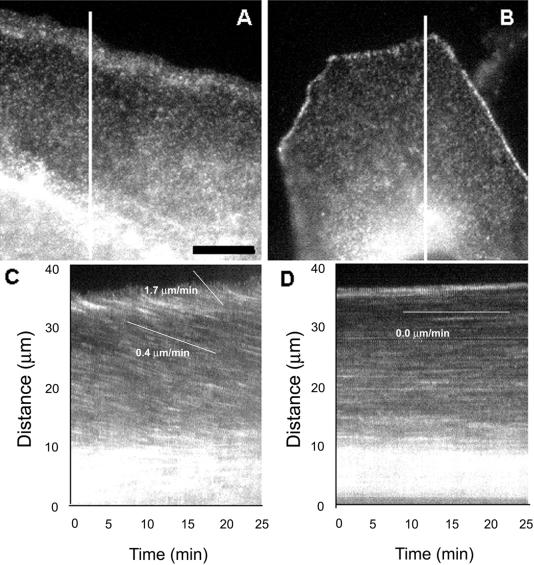
Actin flows rearward in the lamella of migrating cells but not in contacted cells. Cells were microinjected with low levels of X-rhodamine–labeled actin to label actin filaments with fluorescent speckles, and images of the fluorescent actin meshwork were captured at 15-s intervals for 15 min. (A and B) Individual images from the time-lapse series. The low level of fluorescent actin in the cells results in a fluorescence image that exhibits a very fine speckled labeling of the actin meshwork due to stochastic incorporation of labeled and unlabeled actin subunits into actin polymer. The cell in (A) is at the edge of the epithelial cell sheet, with the free leading edge facing the top of the page. Note the concentration of actin polymer in the lamellipodia. The cell in (B) is surrounded on all sides by contacting neighboring cells, which are invisible because they were not injected with fluorescent protein. The cell–cell adhesions are brightly labeled with fluorescent actin. Images of the regions covered by lines in (A) and (B) from each image in the time lapse series were used to construct the kymographs in (C) and (D), respectively. Inhomogeneities in fluorescent labeling of the actin meshwork are seen as streaks across the kymograph. Slopes of the streaks represent velocities of actin meshwork movement. In the free-edged cell in (C), actin moves toward the cell center at 1.7 μm/min in the lamellipodia and at 0.4 μm/min in the lamella. In the cell that is contacted, the actin meshwork remains stationary. Scale bar in (A), 10 μm (equal for all frames).