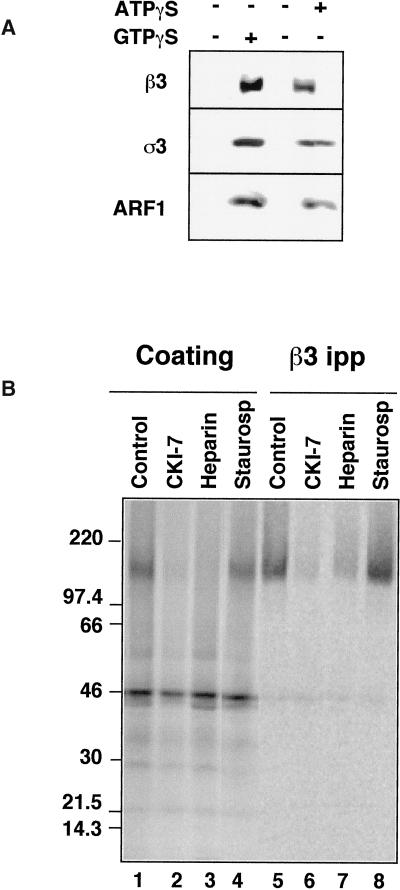
Figure 2.
Recruitment of AP-3 to immobilized synaptic vesicles. (A) AP-3 is recruited to SVs in the presence of ATPγS. Coating assays were performed using unlabeled PC12 N49A synaptic vesicles attached to an antisynaptophysin-protein G-Sepharose affinity column. Reactions were performed with rat brain cytosol, supplemented (lanes 1 and 2) or not (lanes 3 and 4) with an ATP-regenerating system. AP-3 recruitment to SV was induced at 37°C for 30 min either by GTPγS (20 μM, lane 2) or solely by ATPγS (200 μM, lane 4). AP-3 recruitment to synaptic vesicles was assessed by immunoblot with antibodies against either β3 or ς3 subunits of the complex. Similarly, ARF1 binding to membranes was determined with an anti-ARF1 antibody. (B) The β3 subunit of the synaptic vesicle-bound AP-3 complex is thiophosphorylated by acasein kinase activity. Immunoimmobilized PC12 N49A synaptic vesicles were coated in vitro in the presence of dialyzed rat brain cytosol. Coating was triggered by adding [35S]-ATPγS (100 μCi) and warming the reactions at 37°C for 40 min. Reactions were stopped at 4°C, and bound complexes were washed extensively in intracellular buffer. Coated SVs were solubilized from the column by either sample buffer to directly resolve the proteins in SDS-PAGE (lanes 1–4) or in buffer B (lanes 5–8), from which AP-3 β subunits were immunoprecipitated and immunocomplexes were analyzed by SDS-PAGE. The intensity of the β3 thiophosphorylated band was reduced by the casein kinase inhibitors CKI-7 (500 μM, lanes 2 and 6) and heparin (10 μg/ml, lanes 4 and 9) but not by a generic serine–threonine kinase inhibitor staurosporine (10 μM, lanes 4 and 8).