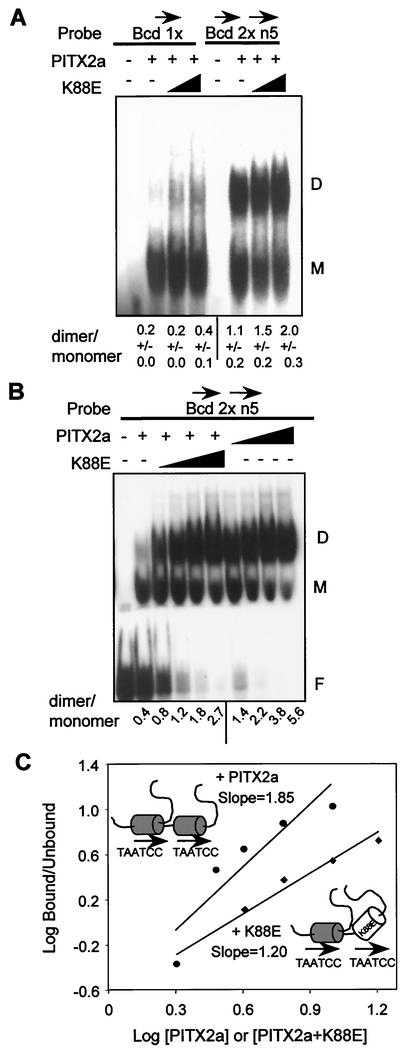
FIG. 6.
The WT-K88E heterodimer binds DNA without cooperativity. (A) EMSA with 500 ng of bacterially expressed PITX2a incubated with 1 pmol of the Bcd 1x or the head-to-tail Bcd 2 × 5n probe in the presence of increasing amounts of K88E (0, 1,000, and 2,000 ng). The monomer and dimer complexes are indicated by the letters M and D, respectively. The amounts of monomer and dimer complexes were determined by using a PhosphorImager system. The data represent mean dimer/monomer ratios ± standard errors of the means from three separate experiments. (B) EMSA with 250 ng of PITX2a incubated with 0.2 pmol of Bcd 2 × 5n probe in the presence of increasing amounts of WT (125, 250, 500, and 1,000 ng) or K88E (250, 500, 1,000, and 2,000 ng) (note that more K88E protein was used). The free probe, monomer, and dimer complexes are indicated. The ratios of dimer to monomer complexes from this representative experiment with both WT and K88E on the same gel are shown. (C) The Hill coefficient was estimated as described in the legend to Fig. 4C for the WT (upper line,circles) and WT plus K88E (lower line, diamonds). The concentrations for WT are expressed in arbitrary units of 2, 3, 4, 6, and 9, representing ∼0.4, 0.6, 0.8, 1.2, and 1.8 μM, respectively, while those for WT-K88E are 2, 4, 6, 9, and 18, representing ∼0.4, 0.8, 1.2, 1.8, 3. and 6 μM, respectively. The WT (shaded homeodomain) and K88E (open homeodomain) protein-DNA complexes are schematically represented.