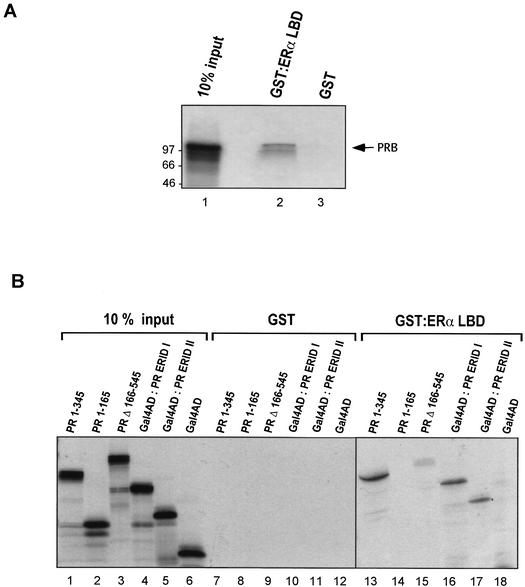
FIG. 5.
Interaction between PRB and ERα in vitro. GST and the GST-ERαLBD fusion protein were expressed in E. coli BL21, bound to glutathione-Sepharose, and incubated with 35S-labeled PRB (wild type or mutants) produced by in vitro transcription and translation in a rabbit reticulocyte lysate. After extensive washing, retained proteins were eluted with SDS loading buffer, separated by SDS-PAGE, and visualized by fluorography. (A) The wild-type PRB interacts with the LBD of ERα in vitro. Lane 1 represents 10% of the amount of 35S-labeled PRB used in the binding assay. The amount of PRB retained by GST-ERαLBD is shown in lane 2. Addition of 1 μM 17β-estradiol, 1 μM R5020, or both together did not alter the amount of PRB retained (data not shown). GST alone did not retain any PRB (lane 3). The positions of molecular mass markers are indicated to the left in kilodaltons. (B) Interaction between mutants of PRB and GST-ERαLBD. The input lanes (lanes 1 to 6) represent 10% of the amount of 35S-labeled PR mutants used in the binding assay. PRB mutants were tested for binding to GST (lanes 7 to 12) and to GST-ERαLBD (lanes 13 to 18).