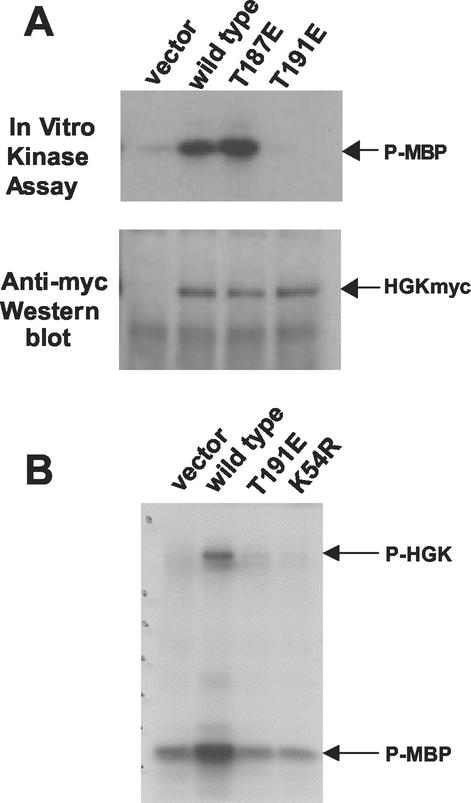
FIG. 3.
Kinase activity of HGK mutants in RIE-1 cells. HGK wild-type and mutant proteins were immunoprecipitated from stable pools of RIE-1 cells infected with HGK retroviruses. (A) Comparison of the T187E and T191E activation loop mutations with wild-type kinase. The top panel shows an autoradiogram of samples analyzed by SDS-PAGE, showing the relative amount of 32P incorporated into myelin basic protein (MBP) after incubation of the immunoprecipitate and [γ-32P]ATP for 20 min at 30οC. The bottom panel shows the relative amount of HGK proteins in the immunoprecipitate. (B) Comparison of the T191E and K54R catalytically inactive HGK mutants with wild-type HGK. An autoradiogram shows the relative amount of 32P incorporation both into HGK protein (autophosphorylation) and into added MBP in the immunoprecipitate.